Ginsenoside-containing vaccine diluent
A technology of ginsenosides and ginseng stem and leaf saponins, which is applied in the field of preparation of vaccine diluents containing ginsenosides, can solve the problems of no immune enhancement effect and weak cellular immune response, so as to improve cellular and humoral immune responses and reduce local irritation , Improving the effect of immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
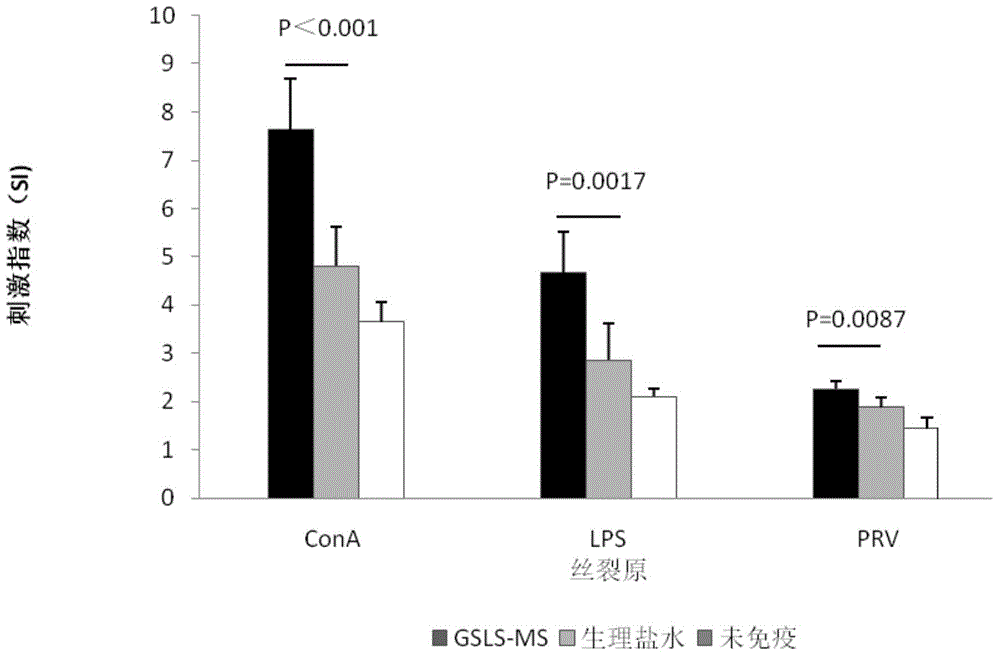
[0035] Embodiment 1: Screening research of vaccine diluent formulation
[0036] 1. Materials and methods
[0037] 1. Reagents
[0038] Thimerosal sodium salt (MS), batch number: F20030210, purchased from China Pharmaceutical Group Shanghai Chemical Reagent Company; sodium chloride injection, batch number: B13020601, purchased from Zhejiang Guojing Pharmaceutical Co., Ltd.; porcine pseudorabies virus gB antibody detection kit , batch number: 09732-FC905, purchased from IDEXX company in the United States; ginseng stem and leaf saponins (GSLS), batch number: HJ140301, total saponin content 80.23%, containing Rg1 6.0%, Re 16.36%, Rbl 2.4%, Rb2 3.8%, Rf 0.1 %, Rc 3.70% and Rd 9%, purchased from Jilin Hongjiu Pharmaceutical Co., Ltd.
[0039] 2. Preparation of vaccine diluent containing ginsenosides and thimerosal
[0040] Preparation of thimerosal sodium salt solution: Weigh a certain amount of thimerosal sodium salt and fully dissolve it in physiological saline for injection to...
Embodiment 2
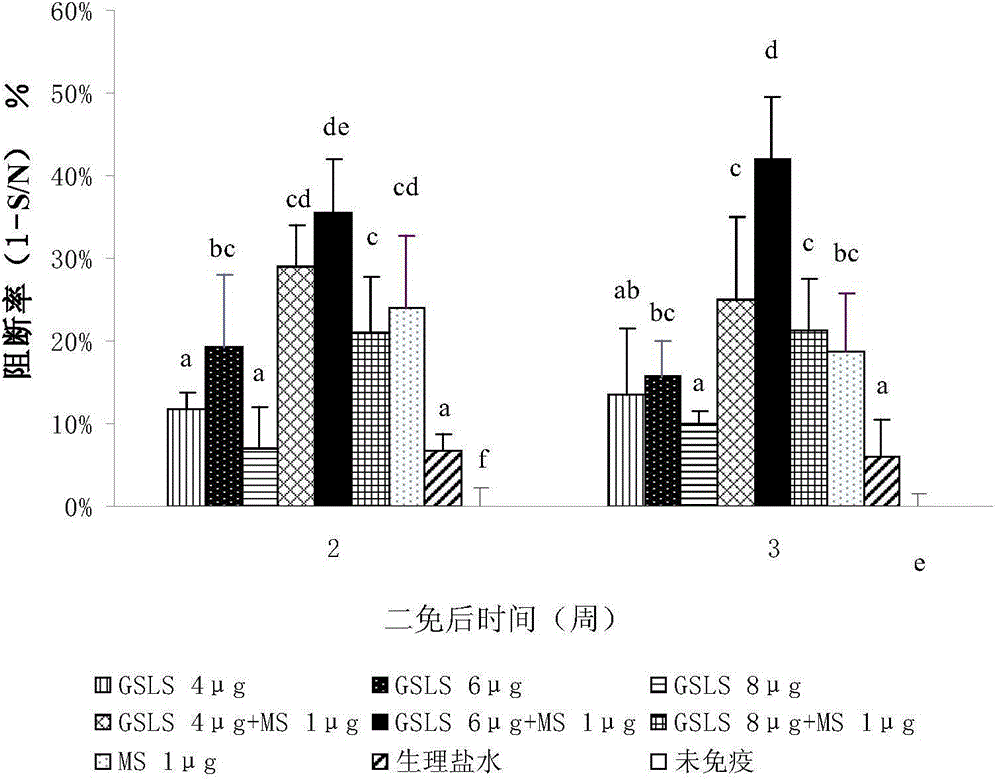
[0065] Example 2: Research on the synergistic adjuvant effect of ginsenosides and thimerosal
[0066] 1. Materials and methods
[0067] 1. Reagent thimerosal sodium salt (MS), batch number: F20030210, purchased from China Pharmaceutical Group Shanghai Chemical Reagent Company; sodium chloride injection, batch number: B13020601, purchased from Zhejiang Guojing Pharmaceutical Co., Ltd.; porcine pseudorabies virus gB antibody Detection kit, batch number: 09732-FC905, purchased from IDEXX company in the United States; ginsenosides (GS), total saponins greater than 90%; containing Rb1 (18.0%), Rb2 (9.5%), Rc (10.0%), Rd (7.6 %), Re (8.7%) and Rg1 (3.5%).
[0068] 2. The preparation of thimerosal sodium salt (MS) solution containing ginsenoside and thimerosal solution: take a certain amount of thimerosal sodium salt and fully dissolve it in normal saline, adjust the solution concentration to 0.02% (weight %), and store at 4°C spare.
[0069] Preparation of ginsenoside (GS) soluti...
Embodiment 3
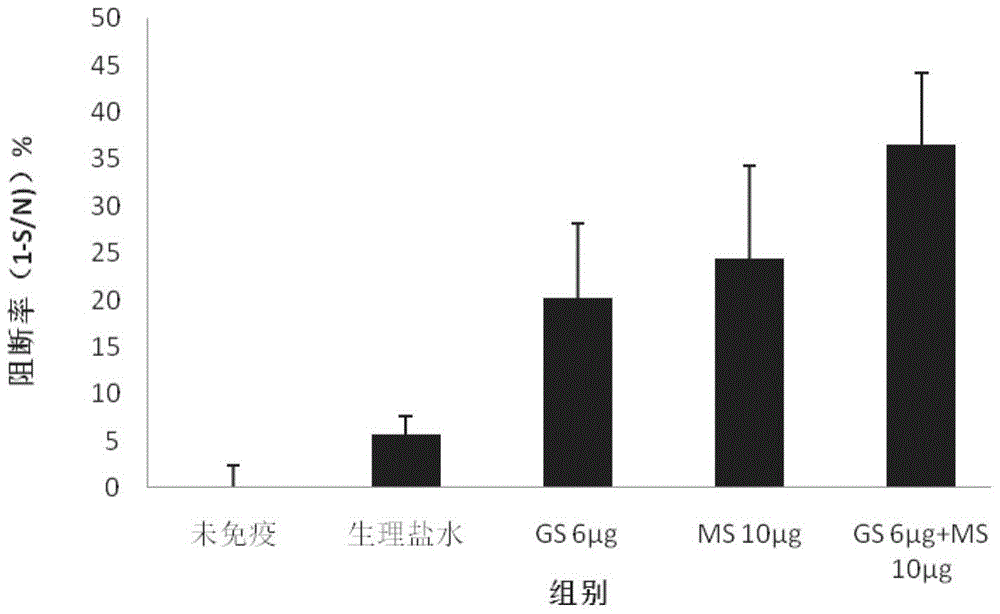
[0088] Example 3: Vaccine diluents enhance serum antibody levels
[0089] 1. Materials and methods
[0090] 1. Reagent thimerosal sodium salt (MS), batch number: F20030210, purchased from China Pharmaceutical Group Shanghai Chemical Reagent Company; sodium chloride injection, batch number: B13020601, purchased from Zhejiang Guojing Pharmaceutical Co., Ltd.; porcine pseudorabies virus gB antibody Detection kit, batch number: 09732-FC905, purchased from IDEXX, USA; ginseng stem and leaf saponins (GSLS): batch number: HJ140301, total saponin content 80.23%, containing Rg1 6.0%, Re 16.36%, Rbl 2.4%, Rb2 3.8% , Rf 0.1%, Rc 3.70% and Rd 9%, purchased from Jilin Hongjiu Pharmaceutical Co., Ltd. HRP-labeled goat anti-mouse IgG1 and IgG2a antibodies, lot number: B13020601, were purchased from Santa Cruz Biotechnology Co., Ltd.
[0091] 2. Preparation of vaccine diluent First prepare 0.002% (weight %) thimerosal normal saline solution as solution A; then prepare 0.012% (weight %) GSLS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com