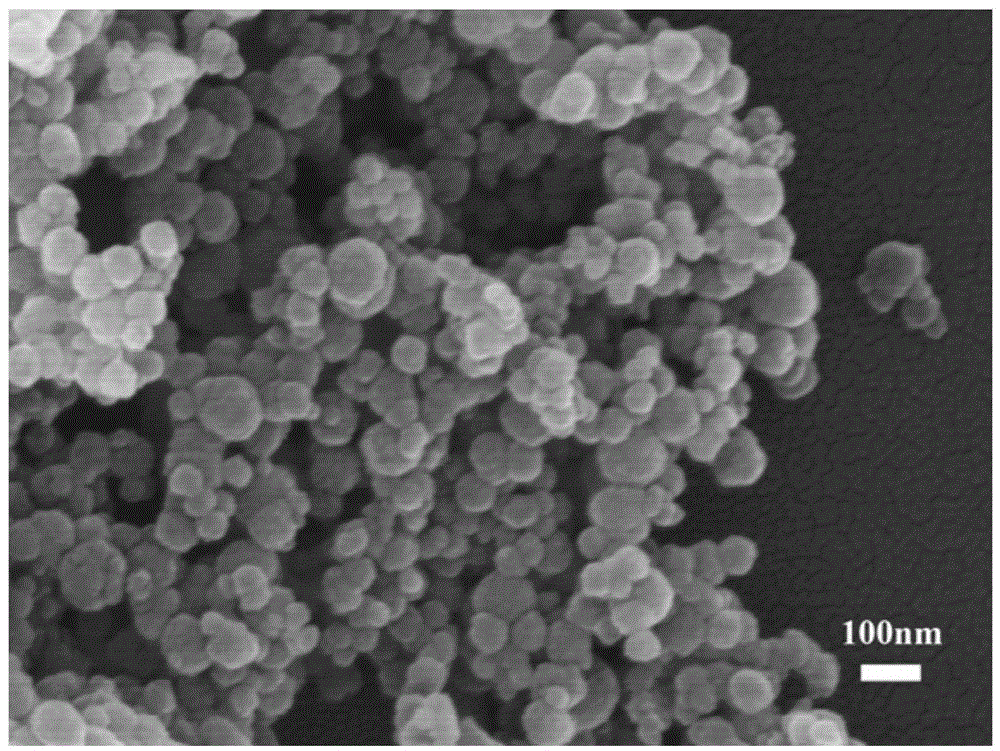
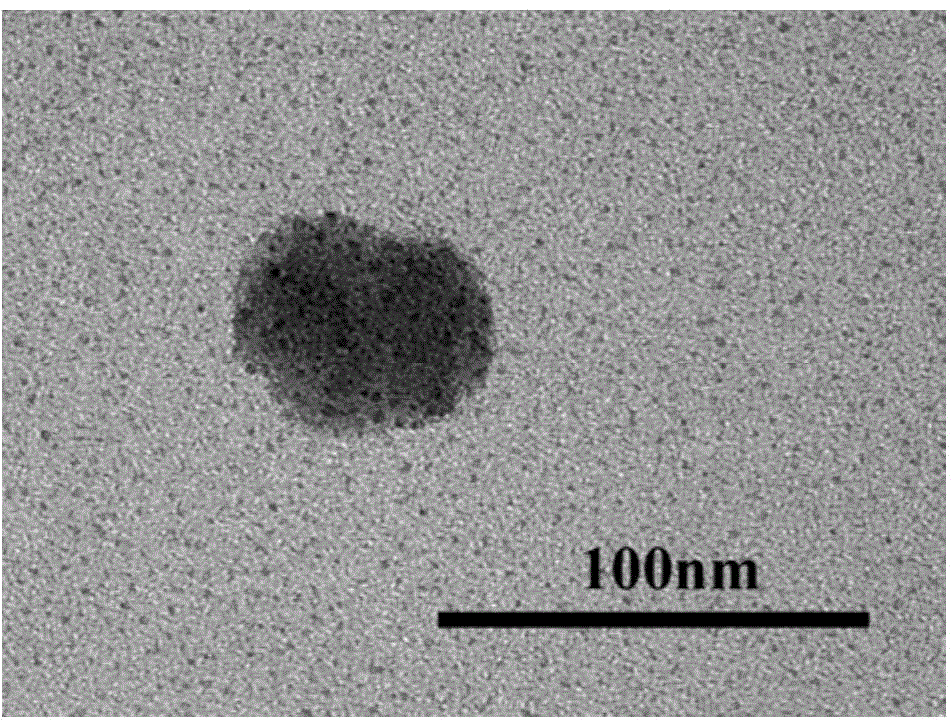
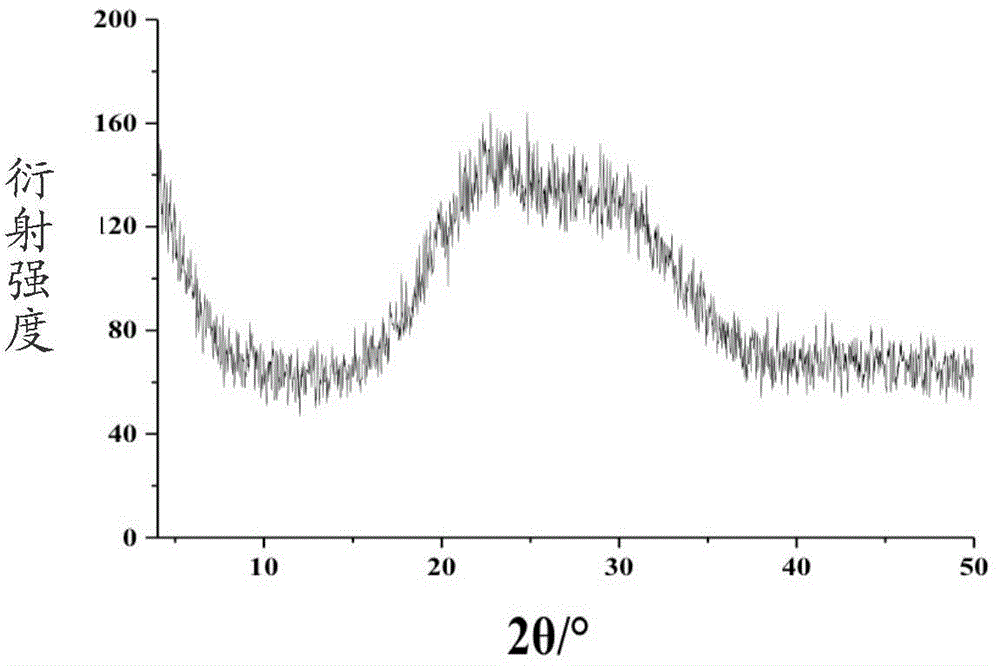
Preparation method for amorphous calcium phosphate nanospheres
A technology of amorphous calcium phosphate and nanospheres, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve problems such as difficulty, and achieve the effect of simple preparation method, easy implementation and small size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1. Preparation of amorphous calcium phosphate nanospheres
[0031] Dissolve a certain quality of calcium chloride and disodium hydrogen phosphate dodecahydrate in 500ml of acetic acid solution with a pH of 2.5, adjust the concentration of calcium chloride to 1mmol / L, and adjust the concentration of disodium hydrogen phosphate dodecahydrate to 0.5mmol / L to obtain a mixed solution of calcium chloride and disodium hydrogen phosphate, then add an aqueous solution of polyacrylic acid to adjust its concentration to 500 μg / ml, and mix evenly to obtain a mixed solution. The solution is sprayed into tiny droplet aerosol through an air compressed atomizer (atomization rate is 0.2mL / min), and the aerosol is blown into a spiral tube at 100°C by using nitrogen at a flow rate of 10L / min as the carrier gas. Tris-HCl buffer solution with a pH value of 8.0 and 0.5 mol / L was used as the receiving solution, and the product precipitate was obtained by filtration; the obtained r...
Embodiment 2
[0034] Embodiment 2. Preparation of amorphous calcium phosphate nanospheres
[0035] Dissolve a certain quality of calcium chloride and disodium hydrogen phosphate dodecahydrate in 500ml of acetic acid solution with a pH of 2.5, adjust the concentration of calcium chloride to 40mmol / L, and adjust the concentration of disodium hydrogen phosphate dodecahydrate to 20mmol / L L, to obtain a mixed solution of calcium chloride and disodium hydrogen phosphate, then add an aqueous solution of polyacrylic acid to adjust its concentration to 500 μg / ml, and mix evenly to obtain a mixed solution. The solution is sprayed into tiny droplet aerosol through an air compressed atomizer (atomization rate is 0.2mL / min), and the aerosol is blown into a spiral tube at 100°C by using nitrogen at a flow rate of 10L / min as the carrier gas. Tris-HCl buffer solution with a pH value of 8.0 and 0.5 mol / L was used as the receiving solution, and the product precipitate was obtained by filtration; the obtained...
Embodiment 3
[0038] Embodiment 3. Preparation of amorphous calcium phosphate nanospheres
[0039] Dissolve a certain quality of calcium chloride and disodium hydrogen phosphate dodecahydrate in 500ml of acetic acid solution with a pH of 2.5, adjust the concentration of calcium chloride to 1mmol / L, and adjust the concentration of disodium hydrogen phosphate dodecahydrate to 1mmol / L L, to obtain a mixed solution of calcium chloride and disodium hydrogen phosphate, then add an aqueous solution of polyacrylic acid to adjust its concentration to 500 μg / ml, and mix evenly to obtain a mixed solution. The solution is sprayed into tiny droplet aerosol through an air compressed atomizer (atomization rate is 0.2mL / min), and the aerosol is blown into a spiral tube at 100°C by using nitrogen at a flow rate of 10L / min as the carrier gas. Tris-HCl buffer solution with a pH value of 8.0 and 0.5 mol / L was used as the receiving solution, and the product precipitate was obtained by filtration; the obtained r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com