Synthetic method of pesticide intermediate 2-chloro-4-formyl valeronitrile
A technology of formylvaleronitrile and dimethylformamide is applied in the field of synthesizing 2-chloro-4-formylvaleronitrile, and can solve the problems of great harm to the environment and operators, complicated preparation process and high quality requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 500ml four-necked round-bottom reaction flask, add 100g of dichloromethane and 0.5g of DMF, purge the system with nitrogen, control the temperature in a water bath, and add 58g of propionaldehyde (1mol) dropwise at 10°C. Chlorine gas is introduced at a flow rate of 16-17L / hr, and the dripping time is 2hr. After the dripping is completed, continue to pass chlorine gas, and keep warm for 2hrs. The liquid is under normal pressure, rectified to recover dichloromethane, and when the liquid temperature reaches 80°C, stop collecting materials. Under the vacuum condition of -0.055MPa, the residue in the still was simply distilled, and 81.8g of distillates with a temperature of 64-66°C were collected.
[0032] Gas spectrum analysis product content is 99.0%, appearance is colorless and transparent, and the yield of 2-chloropropionaldehyde is 87.5%.
Embodiment 2-4
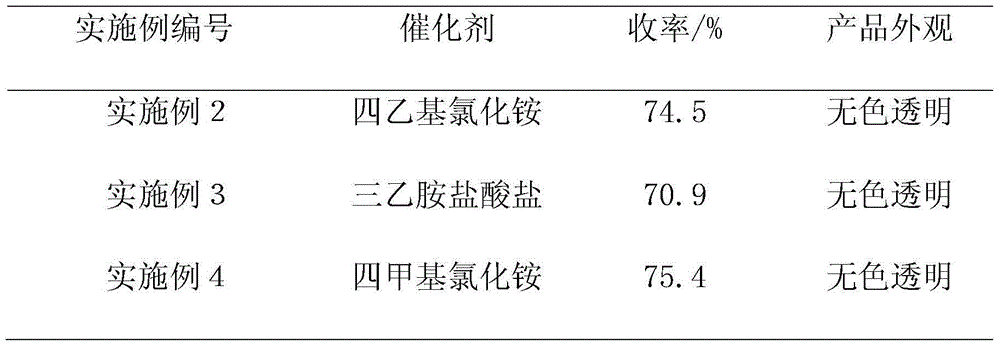
[0034] On the basis of Example 1, tetraethylammonium chloride, triethylamine hydrochloride, and tetramethylammonium chloride were used as catalysts respectively, and other conditions remained unchanged. The obtained results are shown in Table 1.
[0035] The impact of different catalysts in table 1 on the yield of 2-chloropropionaldehyde
[0036]
Embodiment 5-7
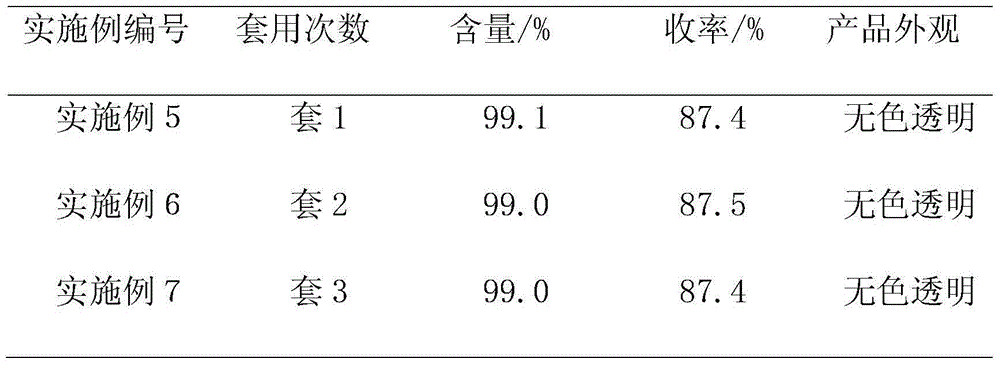
[0038] The methylene chloride recovered in Example 1 is applied mechanically, and other conditions are the same as in Example 1. The obtained results are shown in Table 2.
[0039] Table 2 applies mechanically to the impact of reclaiming dichloromethane on 2-chloropropionaldehyde
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com