Synthetic method for ultraviolet absorbent--octyl triazone
A technology of octyl triazone and synthesis method, which is applied in the field of synthesis of triazine ultraviolet absorber octyl triazone, and achieves the effects of advanced technology, high liquid phase purity and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of synthetic method of ultraviolet absorber octyl triazone, this method is made up of the following steps:
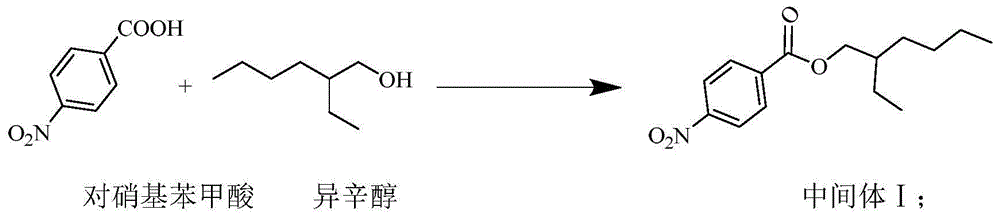
[0025] Synthesis of Intermediate I
[0026] Add 334.26g (1.0mol) of p-nitrobenzoic acid, 195.34g (1.5mol) of isooctyl alcohol, 16.70g (0.14mol) of sodium bisulfate, and 600mL of toluene into a reaction flask equipped with a water separator, at 135-145°C React for 6-7 hours until no water comes out. After cooling to room temperature, add 200mL distilled water and stir for 15min, then let stand to separate the organic phase. The toluene was distilled off under reduced pressure to obtain intermediate I with a yield of 89.2%.
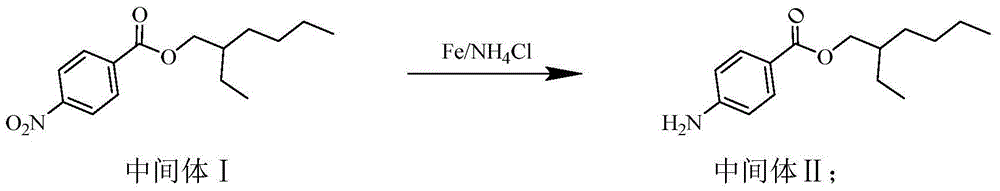
[0027] Synthesis of Intermediate II
[0028] In the three-necked flask, add 920mL methanol-water solution (V 甲醇 :V 水 =5:1), 48.15g (1.8mol) of ammonium chloride, 33.60g (1.2mol) of reduced iron powder and 55.87g (0.20mol) of intermediate I, stirred and heated to reflux for 16-17h. TLC followed the reaction process. After the reacti...
Embodiment 2
[0035] Synthesis of Intermediate I
[0036] The amount of iso-octanol is increased to 71.4g (0.27mol), the others are the same as Example 1, and the yield of intermediate I is 92.5%.
[0037] Synthesis of Intermediate II
[0038] The amount of reduced iron powder is increased to 89.60g (1.6mol), the amount of ammonium chloride is increased to 107.0g (2.0mol), others are the same as Example 1, and the yield of intermediate II is 93.2%.
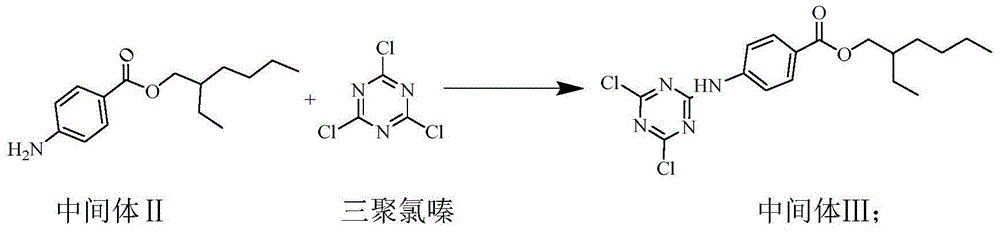
[0039] Synthesis of Intermediate III
[0040] Increase the amount of intermediate II to 115.84 g (0.44 mol), and the others are the same as Example 1, and the yield of intermediate III is 93.2%.
[0041] Synthesis of Octyl Triazone
[0042] Increase the amount of intermediate II to 121.11 g (0.46 mol), and the others are the same as Example 1, and the yield of octyl triazone is 91.6%.
Embodiment 3
[0044] Synthesis of Octyl Triazone
[0045] Increase the amount of intermediate II to 126.37g (0.48mol), and the others are the same as Example 1, and the yield of octyl triazone is 93.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com