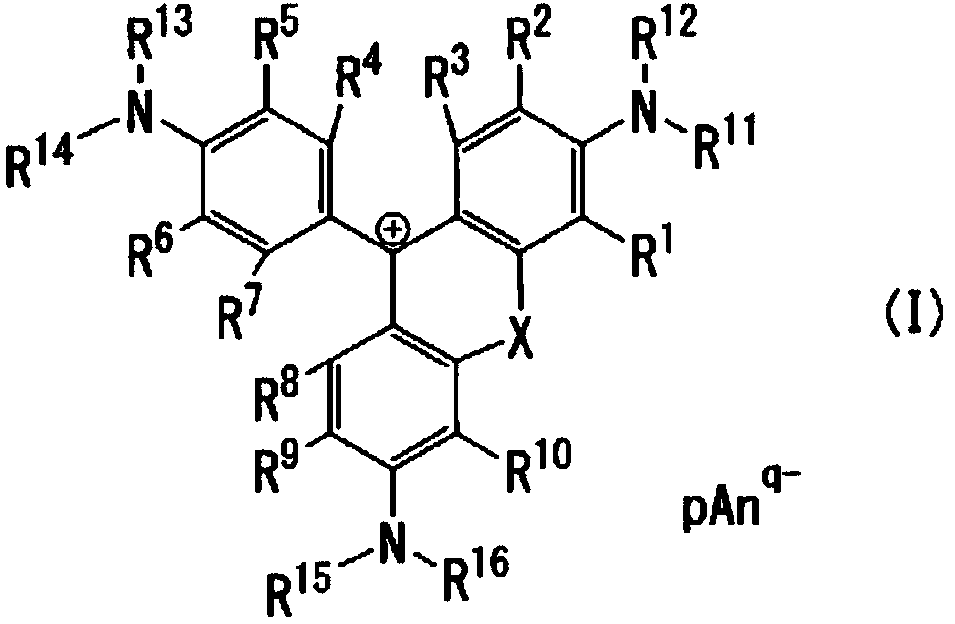
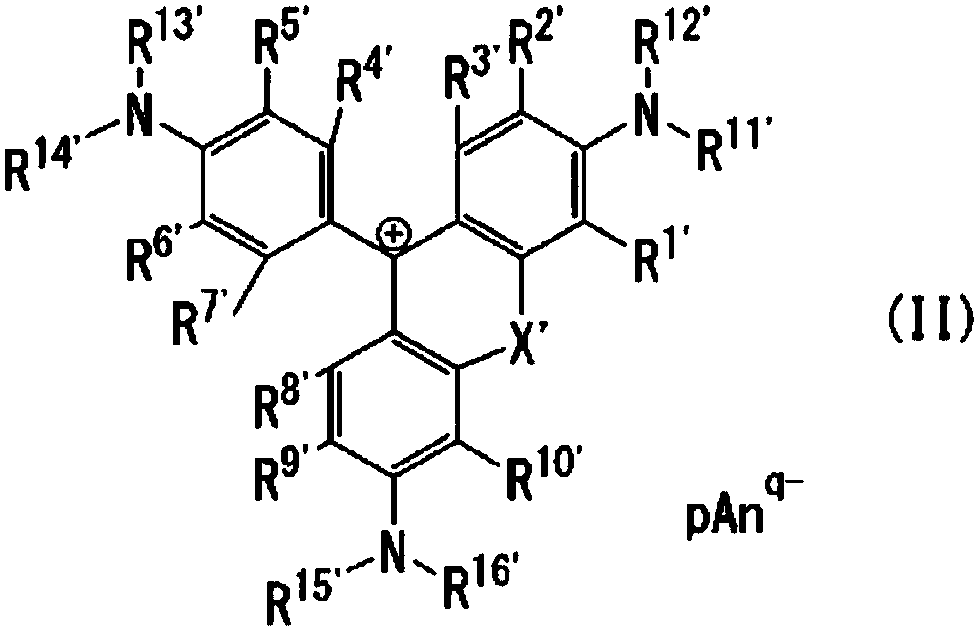
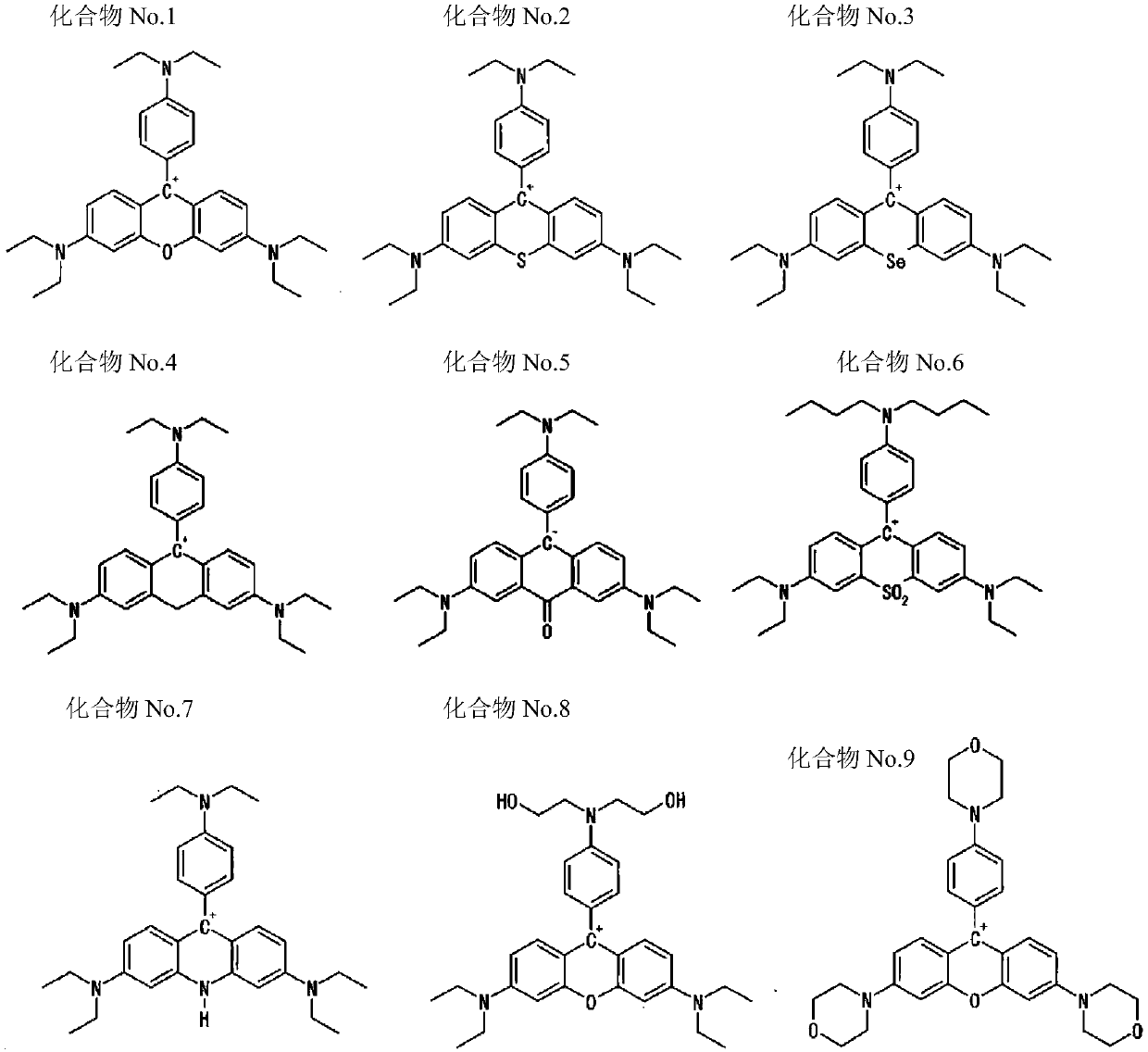
Coloring photosensitive compositions and compounds
A technology of photosensitive composition and polymeric compound, which is applied in optics, optical filters, optical components, etc., can solve problems such as easy color mixing, triarylmethane pigment heat resistance that cannot meet the requirements, and no records, etc., to achieve The effect of excellent solubility and heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0160] [Example 1-1] Bis(trifluoromethanesulfonyl)imide ((CF) of compound No.25 3 SO 2 ) 2 N - ) synthesis of salt
[0161]
[0162] 0.01 mol of compound S-1 represented by the following chemical formula, 0.02 mol of diethylaminophenol, and 0.03 mol of sulfuric acid were mixed, and stirred at 140° C. for 8 hours. The reaction solution was added dropwise to an aqueous sodium hydroxide solution, the organic matter was extracted with toluene and washed with water three times, and the solvent was distilled off from the organic layer. The residue was purified by silica gel column chromatography (developing solvent: chloroform) to obtain 0.005 mol of a colorless product (52% yield).
[0163] Compound S-1
[0164]
[0165]
[0166] 0.05 mol of the colorless product obtained in Step 1, 0.75 mol of chloranil, 0.25 mol of acetic acid, and 0.025 mol of hydrochloric acid were mixed and stirred at 60°C for 1 hour. The solid was filtered off, chloroform was added to the filtrat...
Embodiment 1-2~1-4
[0168] [Examples 1-2 to 1-4] Synthesis of bis(trifluoromethanesulfonyl)imide salts of compounds No.43, No.50 and No.51
[0169] Bis(trifluoromethanesulfonyl)imide salts of compounds No.43, No.50 and No.51 were synthesized according to the method of Example 1-1, and various analyzes were performed to confirm that they were the target compounds. The analysis results are shown in Table 1 and Table 2.
[0170] Table 1
[0171]
[0172] Table 2
[0173]
Embodiment 2-1~2-5 and comparative example 2-1~2-2
[0174] [Examples 2-1 to 2-5 and Comparative Examples 2-1 to 2-2] Colored alkali-developable photosensitive compositions No.1 to No.5 and comparative colored alkali-developable photosensitive compositions No.1 to Preparation of No.2
[0175] Preparation of Alkali Developable Photosensitive Composition
[0176] 30.33 g of ACA Z251 (manufactured by DAICEL CYTEC Co., Ltd.) and 11.04 g of Aronix M-450 (manufactured by Toagosei Co., Ltd.) as the component (B), 1.93 g of IRGACURE907 (manufactured by BASF Co., Ltd.) as the component (C), and PGMEA36 as the solvent .60 g and 0.01 g of FZ2122 (manufactured by Dow Corning Toray Co., Ltd.) as other components were mixed and stirred until the insoluble matter disappeared to obtain an alkali-developable photosensitive composition.
[0177] Preparation of dye solutions No.1 to No.5 and comparative dye solutions No.1 to No.2
[0178] 0.10 g of each compound described in Table 3 and PGMEA 1.90 g as (A) component were added, stirred and dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com