Adenosine receptor stimulating agent and application thereof
An adenosine receptor and reagent technology, applied in the field of adenosine A1 receptor agonists, can solve problems such as hindering the use and unstable metabolism of adenosine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
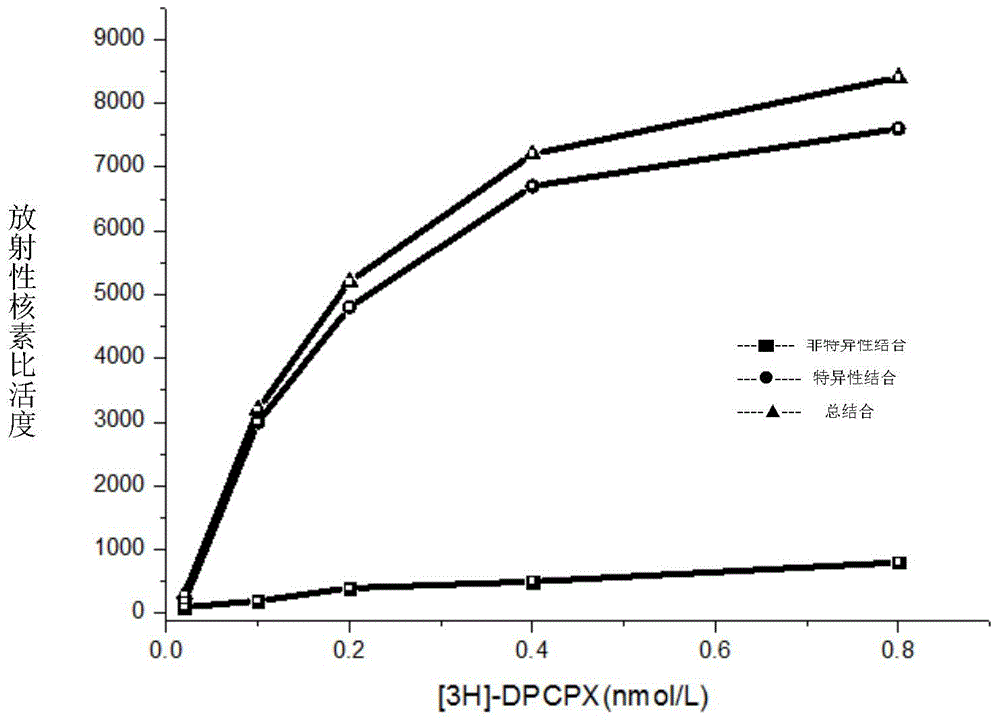
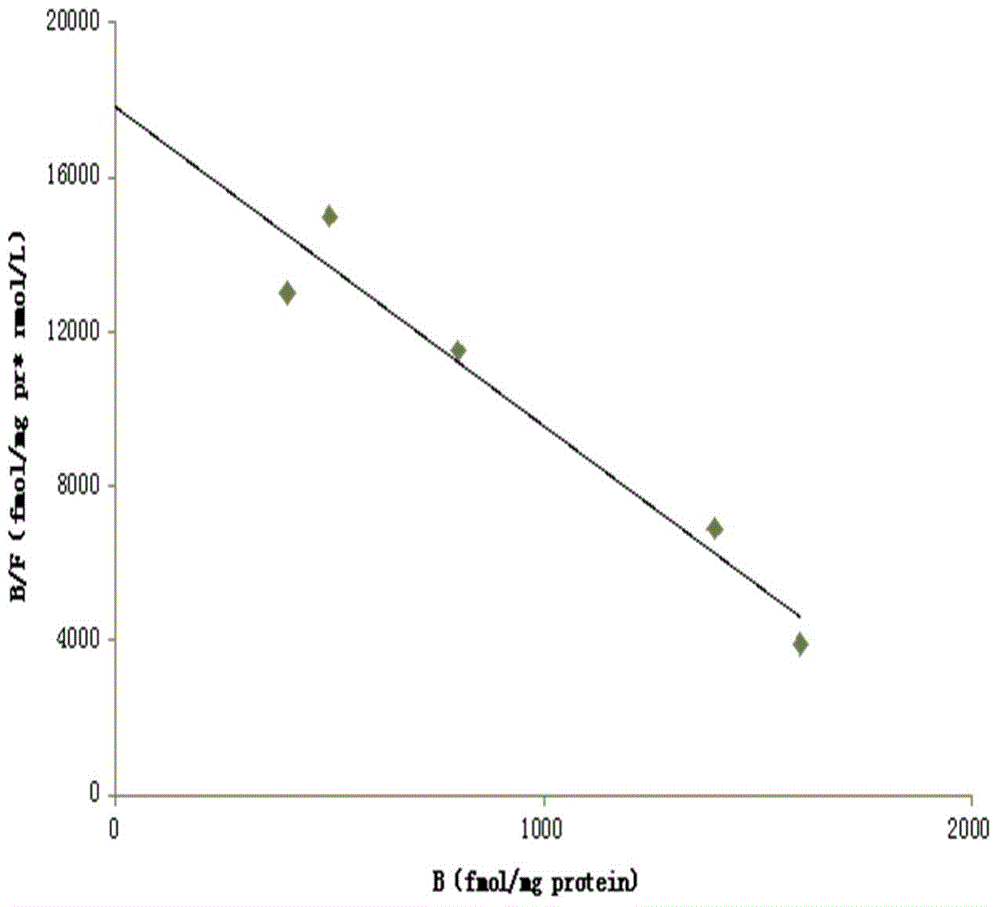
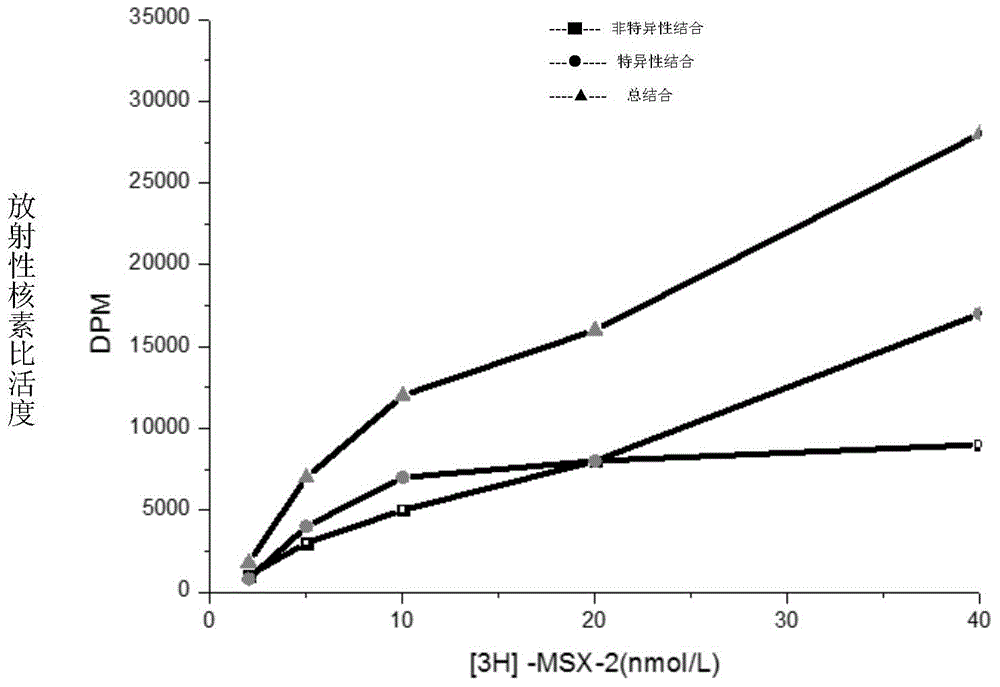
[0077] 1.HEA is A 1 selective agonist
[0078] 1.1 Preparation of membrane receptor protein
[0079] The brains of Wistar rats were decapitated, the cerebral cortex and striatum were separated, weighed separately, and 10 times the volume of ice-cold Tris-HCL buffer (50mM, pH7.5) was added at a ratio of 1:10, the tissue was homogenized, and mixed. After the suspension was centrifuged, the supernatant was discarded, and after washing with the above solution for 3 times, the supernatant was discarded by centrifugation again, and the precipitate was re-mixed with 50mM Tris-HCL buffer, and the rat cerebral cortex protein was determined by the Coomassie brilliant blue method (Bradford method). The concentration is 0.8mg / ml, and the protein content of rat striatum brain tissue homogenate is 1.3mg / ml. Store at -80°C after aliquoting. (Li M, Kang RX, Shi JG, Liu GT, Zhang JJ, 2013. Anticonvulsant Activity of B2, an Adenosine Analog, on Chemical Convulsant-Induced Seizures, PLo...
Embodiment 2
[0089] Example 2. Application of HEA in anticonvulsant
[0090] 2.1 Animal model and administration method
[0091] Male ICR mice, 18-22 g, were purchased from the Animal Experiment Center of Wenzhou Medical College. The animals were acclimated to the environment for at least 5 days before the experiment. The room temperature was maintained at 25°C, with free access to food and water. Healthy male ICR mice were randomly divided into control group (1% DMSO, ip), model group and CCPA group (0.1 mg·kg -1 ,ip), HEA group (15mg / kg, 40mg / kg, 60mg / kg), DPCPX group (2mg·kg -1 , ip), ZM241385 group (1mg·kg -1 , 5mg·kg -1 , ip), DPCPX+HEA (2mg·kg -1 +40mg / kg, ip) group and ZM241385+HEA (1mg·kg -1 +40mg / kg, 5mg·kg -1 +40mg / kg ip) group. Adenosine A 1 R receptor antagonist DPCPX (or A 2 R receptor antagonist ZM241385) was given intraperitoneal injection 10 minutes before administration, and pentylenetetrazol (100 mg·kg -1 , ip) induced convulsions in mice; in the antagonist gr...
Embodiment 3
[0096] Example 3. Application of HEA in cerebral ischemia
[0097] 3.1 Animal model and administration method
[0098] A rat model of cerebral ischemia with distal middle cerebral artery occlusion was used. ① The rats were anesthetized by intraperitoneal injection with 10% chloral hydrate (3ml / kg) according to their body weight. ②Line the rat on the right side and fix it, make a 1cm skin incision at the line between the inner canthus and the external auditory canal, separate the fascia and muscle tissue, and expose the skull; ③Use a cotton ball to suck a small amount of saline to wipe the skull until the vision is clear;④ Under the operating microscope, the fascia was separated, the skull was exposed, and a 2mm diameter hole was drilled near the upper 1 / 3 of the bone ridge; ⑤The excess bone debris was washed with a small amount of normal saline, and the meninges were removed to expose the middle cerebral artery MCA; ⑥The Rats were fixed in the supine position, and the cent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com