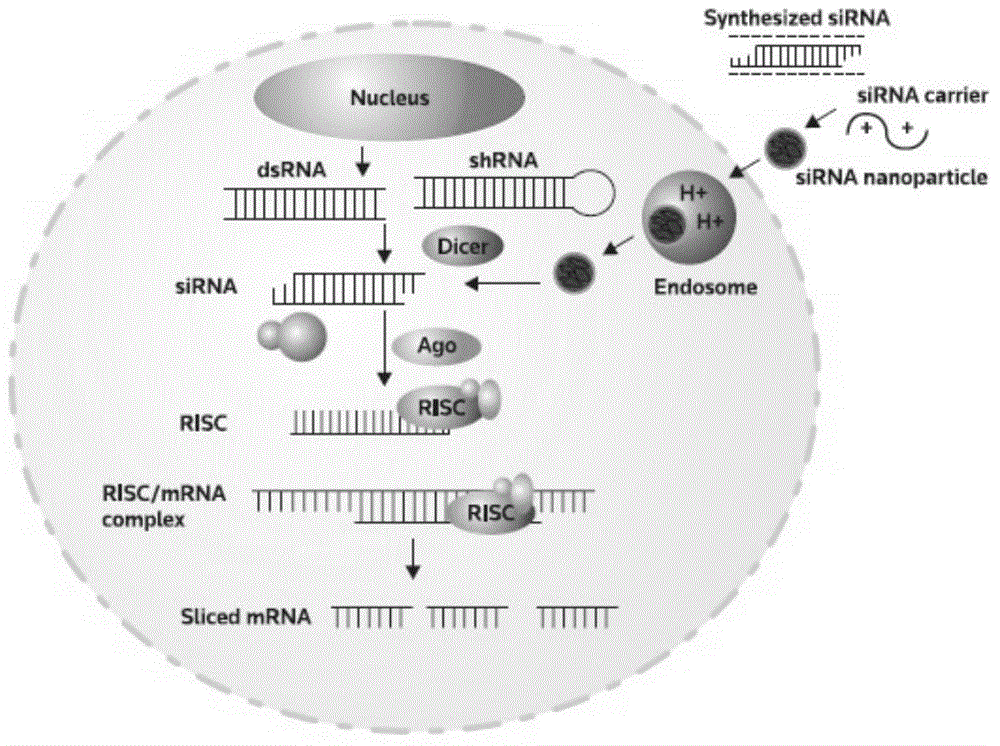
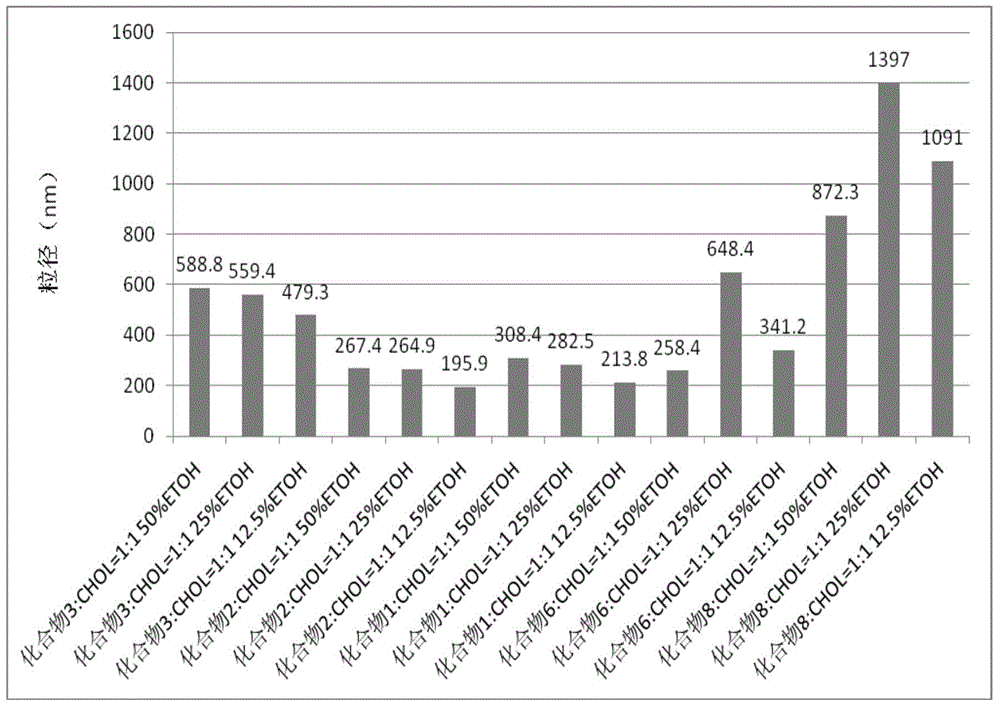
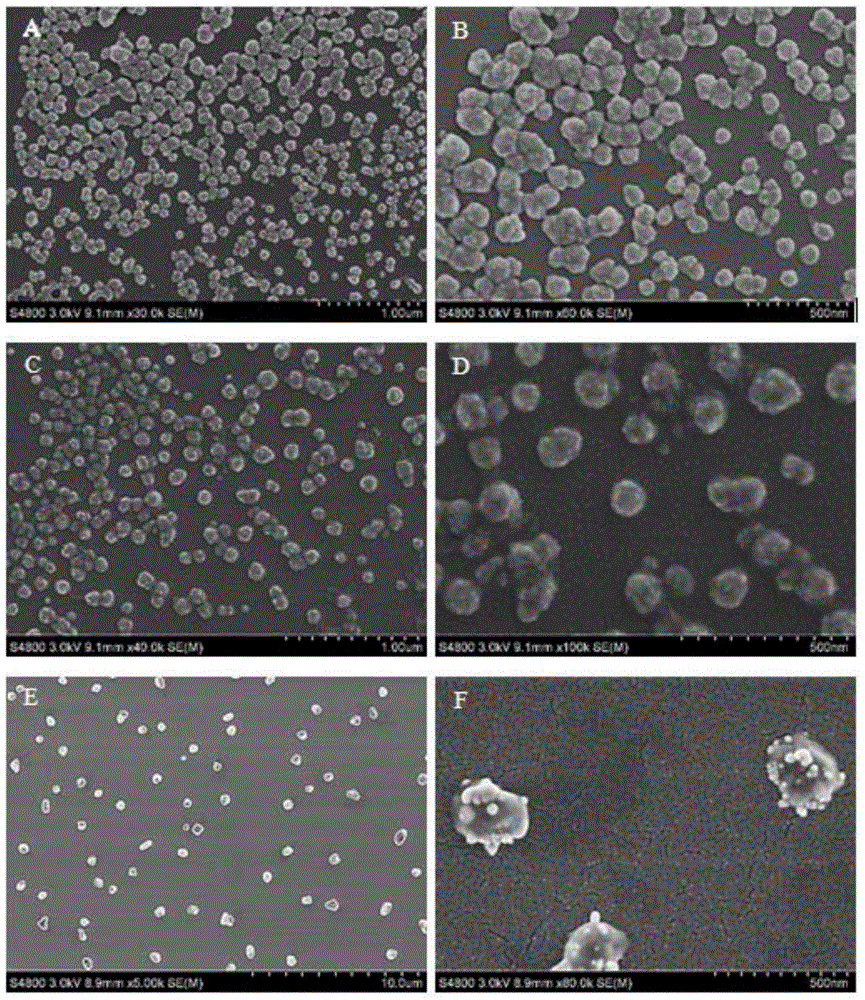
Liposome-modified spermine derivative and liposome prepared by derivative
A technology of lipid modification and derivatives, which is applied in the preparation of carbamic acid derivatives, liposome delivery, carboxylic acid amide preparation, etc., can solve the problems of not reaching the therapeutic effect, and achieve long cycle performance and targeting, The effect of strengthening market competitiveness and improving accumulation capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Compound 1 was synthesized.
[0047] Weighed 100 mg of compound 12 into a 100 ml round bottom flask, added 3 ml of trifluoroacetic acid, stirred at room temperature for 12 hours, and evaporated the trifluoroacetic acid to dryness. The pre-treated ion exchange resin is used to pass through the column, and the obtained liquid is freeze-dried to obtain the obtained product. Yield 98%.
[0048] 1H NMR (400MHz, CDCl 3 ):δ=0.880(t,6H,j1=7.2Hz,j2=6.4Hz),1.25-1.33(m,44H),1.41-1.57(m,10H),1.96-2.00(m,10H),2.63- 3.10(m,12H),5.32-5.38(m,4H);13C NMR(400MHz,CDCl 3 ): δ=14.147, 22.710, 27.256, 29.347, 29.563, 29.798, 31.930, 129.744, 129.777, 130.006, 130.044. HRMS (MALDI) found 731.7142 [M + H] + (Calculated mass for C 46 h 90 N 4 o 2 was 731.7142[M+H]+).
[0049] The reaction scheme is as follows:
[0050]
Embodiment 2
[0052] Compound 2 was synthesized.
[0053] Weighed 100 mg of compound 16 into a 100 ml round bottom flask, added 3 ml of trifluoroacetic acid, stirred at room temperature for 12 hours, and evaporated the trifluoroacetic acid to dryness. The pre-treated ion exchange resin is used to pass through the column, and the obtained liquid is freeze-dried to obtain the obtained product. Yield 98%.
[0054] 1 H NMR (400MHz, DMSO-d6): δ = 0.835-0.868 (t, 6H, j1 = 7.2, j2 = 6), 1.236-1.403 (m, 48H), 1.530-1.563 (m, 8H), 1.956-1.986 (m,8H),2.321-2.397(m,12H),2.621-2.715(m,8H),3.347-3.558(m,4H),3.963-3.996(t,4H,j1=6.4,j2=6.8), 5.321-5.357 (m, 2H).
[0055] 13 C NMR (400MHz, CDCl 3 ): δ=14.143, 22.711, 25.764, 27.244, 29.260-29.797, 31.933, 32.838, 63.122, 129.987. HRMS (ESI) found 847.8009[M+H]+(Calculated mass for C 52 h 102 N 4 o 4 was 847.7936[M+H]+).
[0056]
Embodiment 3
[0058] Compound 3 was synthesized.
[0059] Weighed 100 mg of compound 11 into a 100 ml round bottom flask, added 3 ml of trifluoroacetic acid, stirred at room temperature for 12 hours, and evaporated the trifluoroacetic acid to dryness. The pre-treated ion exchange resin is used to pass through the column, and the obtained liquid is freeze-dried to obtain the obtained product. Yield 98%.
[0060] 1 H NMR (400MHz, CDCl 3 ):δ=0.880(t,6H,j1=7.2Hz,j2=6.4Hz),1.26-1.41(m,44H),1.58(br,8H),1.90-2.0(m,8H),2.12-2.27( m, 4H), 2.92 (br, 4H), 3.30-3.56 (m, 8H), 5.30-5.38 (m, 4H).
[0061] 13 C NMR (400MHz, CDCl 3 ): δ=14.138, 22.711, 27.189, 27.245, 29.283, 29.352, 29.529, 29.585, 29.728, 29.801, 31.937, 52.553, 53.464, 129.794, 129.952. C 46 h 94 N 4 was703.7557[M+H]+).
[0062] The reaction scheme is as follows:
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com