Method for cumyl-hydroperoxide-propylene-oxide (CHPPO)-based propylene oxide generating device with by-product dicumyl peroxide (DCP)
A technology for the by-production of dicumyl peroxide and propylene oxide, which is applied to the preparation of peroxide compounds, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as high energy consumption and material consumption, and generation of sulfide wastewater
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
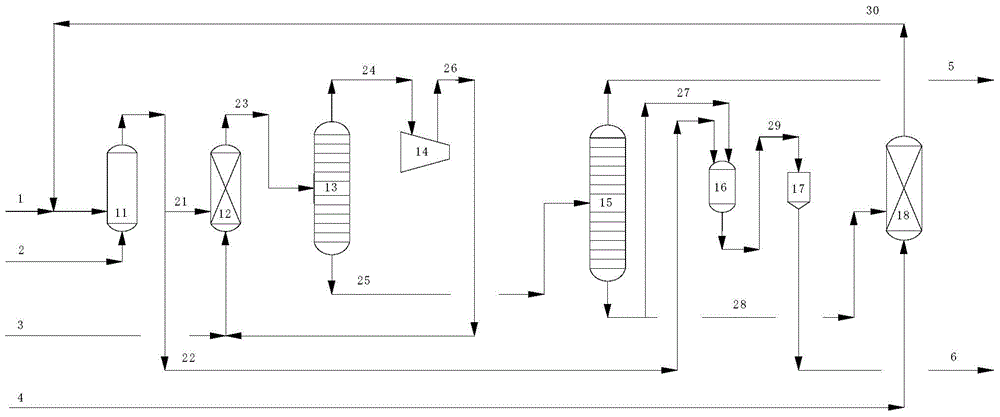
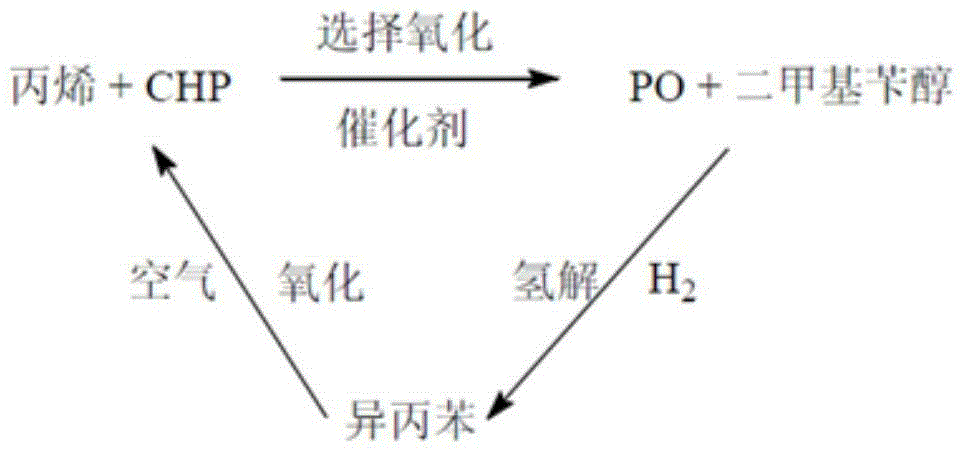
[0027] A method for producing the main product of propylene oxide PO and the by-product of dicumyl peroxide DCP by adopting the steps of oxidation reaction, epoxidation reaction, condensation reaction, hydrogenolysis reaction and the like described in the present invention.
[0028] The operating conditions of the cumene oxidation reactor are: oxidation temperature 90°C, oxidation pressure 0.30MPaG; epoxidation reaction conditions are: epoxidation temperature 60°C, epoxidation pressure 5.5MPaG; condensation reactor operating conditions It is: the condensation temperature is 40°C, the condensation pressure is 0.002MPaG; the hydrogenolysis reaction conditions are: the hydrogenolysis temperature is 185°C, and the hydrogenolysis pressure is 2.2MPaG. Catalyst A is Ti-HMS catalyst; acid catalyst B is perchloric acid catalyst; catalyst C is Pd-Ni-Mg-SiO 2 catalyst. The mass ratio of CHP material to propylene in the epoxidation reaction is 1:1.42; the mass ratio of one CHP material t...
Embodiment 2
[0033] According to the conditions and steps described in Example 1, only the production scale of DCP was changed to 20,000 tons / year. The flow ratio of dimethyl benzyl alcohol CA intermediate product participating in condensation reaction and hydrogenolysis reaction is 1:23.3, the circulation of CA is 224,400 tons / year, and the consumption of hydrogen is 3993 tons / year. As a result, the circulation of CA is reduced by 10,100 tons / year, and the consumption of hydrogen is reduced by 179.3 tons / year; since there is no reduction reaction in the production of DCP, there is no need to consume sulfide reducing agents and no sulfide wastewater is generated.
Embodiment 3
[0037] According to the conditions and steps described in Example 1, only the production scale of DCP was changed to 30,000 tons / year. The flow ratio of dimethyl benzyl alcohol CA intermediate product participating in condensation reaction and hydrogenolysis reaction is 1:15.5, the circulation volume of CA is 219,400 tons / year, and the consumption of hydrogen gas is 3904 tons / year. As a result, the circulation of CA is reduced by 15,100 tons / year, and the consumption of hydrogen is reduced by 268.9 tons / year; since there is no reduction reaction in the production of DCP, there is no need to consume sulfide reducing agents, and no sulfide wastewater is generated.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com