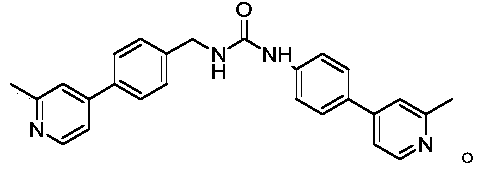
Wnt signal channel inhibitor and application thereof
A technique for signaling pathways and inhibiting activity, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 This example provides a synthetic method for heterocyclic compounds B1-B32
[0048] (1) Heterocyclic compound B1, which is synthesized by the following method:
[0049]
[0050] 1) Synthesis of Intermediate B1-2:
[0051] Dissolve B1-1 (1.2g, 6.98mmol) and triethylamine (800mg, 8.37mmol) in dichloromethane (30mL), slowly add chloroacetyl chloride (960mg, 8.37mmol) under ice-cooling, and stir at room temperature for 12 hours, add water (50mL), extract with ethyl acetate (50mL*3), combine the organic phases, wash with saturated brine (30mL*2), dry over anhydrous sodium sulfate, concentrate under reduced pressure and go through column chromatography (petroleum Ether:ethyl acetate=3:1) to obtain a pale yellow solid (800mg, 47%).
[0052] 2) Synthesis of Intermediate B1-3:
[0053] B1-2 (120 mg, 0.48 mmol) and p-bromophenol (92 mg, 0.53 mmol) were dissolved in acetone (8 mL), and potassium carbonate (80 mg, 0.58 mmol) and potassium iodide (8 mg, 0.048 mmol) w...
Embodiment 2
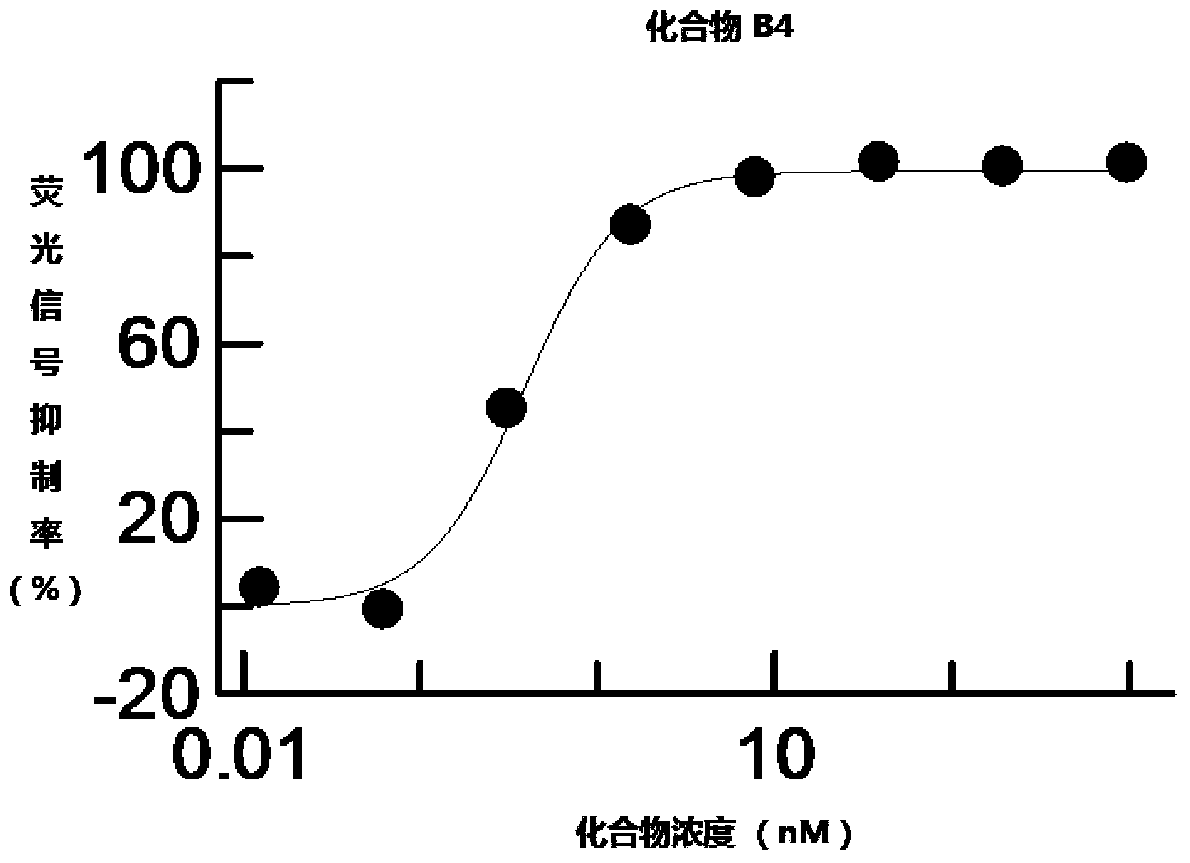
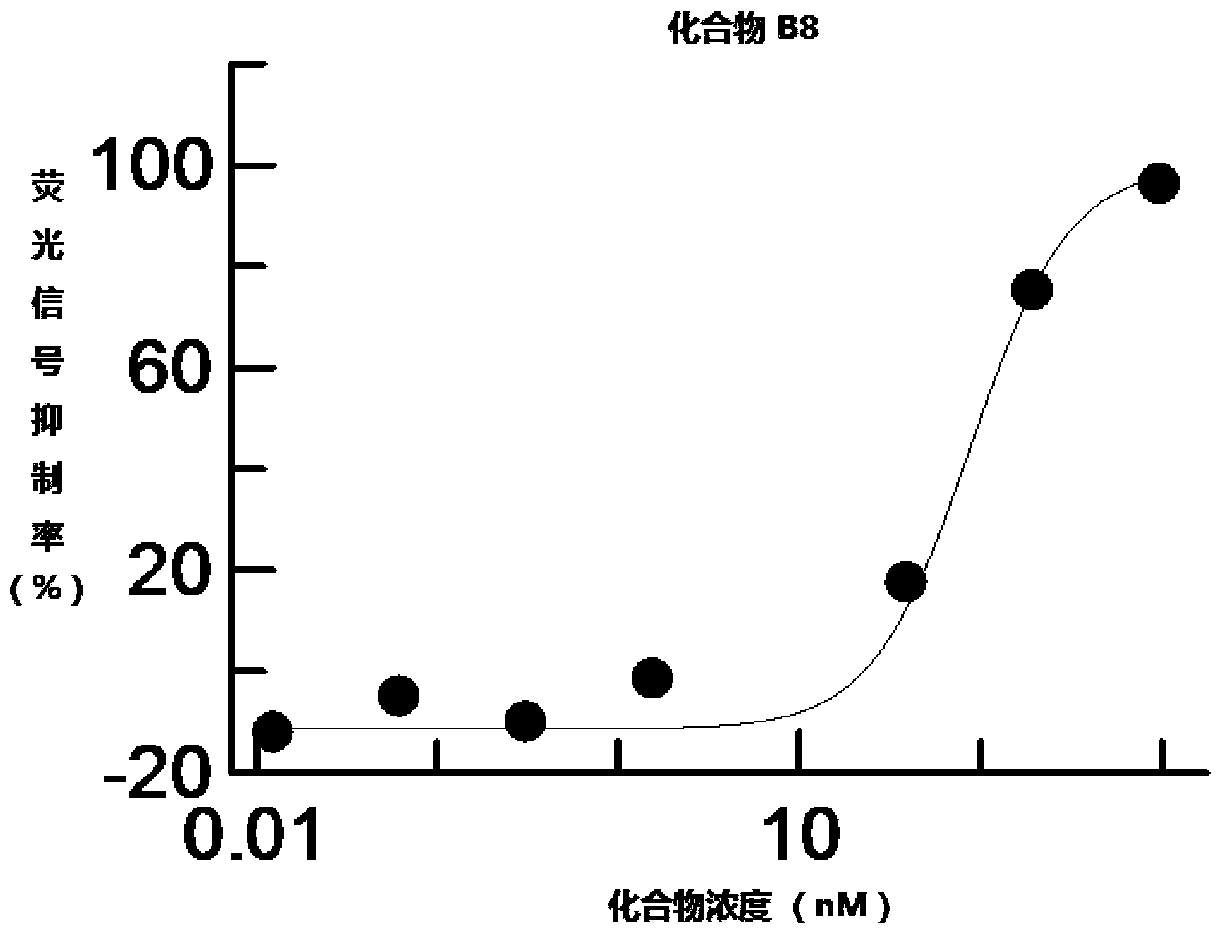
[0238] Example 2 Determination of Inhibition Ability of Heterocyclic Compounds B1-B32 to Wnt Signaling Pathway
[0239] L Wnt3A cells (CRL-2647, ATCC) were cultured in DMEM medium (Gibico) containing 10% fetal bovine serum (Hyclone). HEK293 STF stable clone cells (HEK293 cells transfected with "Super-Top Flash" TCF fluorescent reporter plasmid) were cultured in complete medium (containing 4mM L-glutamine, 1.5g / L sodium bicarbonate, 4.5g / L glucose , 6 μg / mL blasticidin and 10% fetal bovine serum in DMEM medium). When L Wnt3A cells and HEK293 STF stable clone cells were cultured to 90% confluency, they were harvested separately and mixed at a ratio of 1:1. 100 μL / well of the mixed cell culture solution was added to a 96-well plate to make the final cell concentration 12,000 cells / well, and then cultured for another 24 hours. The compounds to be tested were serially diluted with DMSO, and then diluted to the desired concentration with DMEM medium. 20 μL of the compound solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com