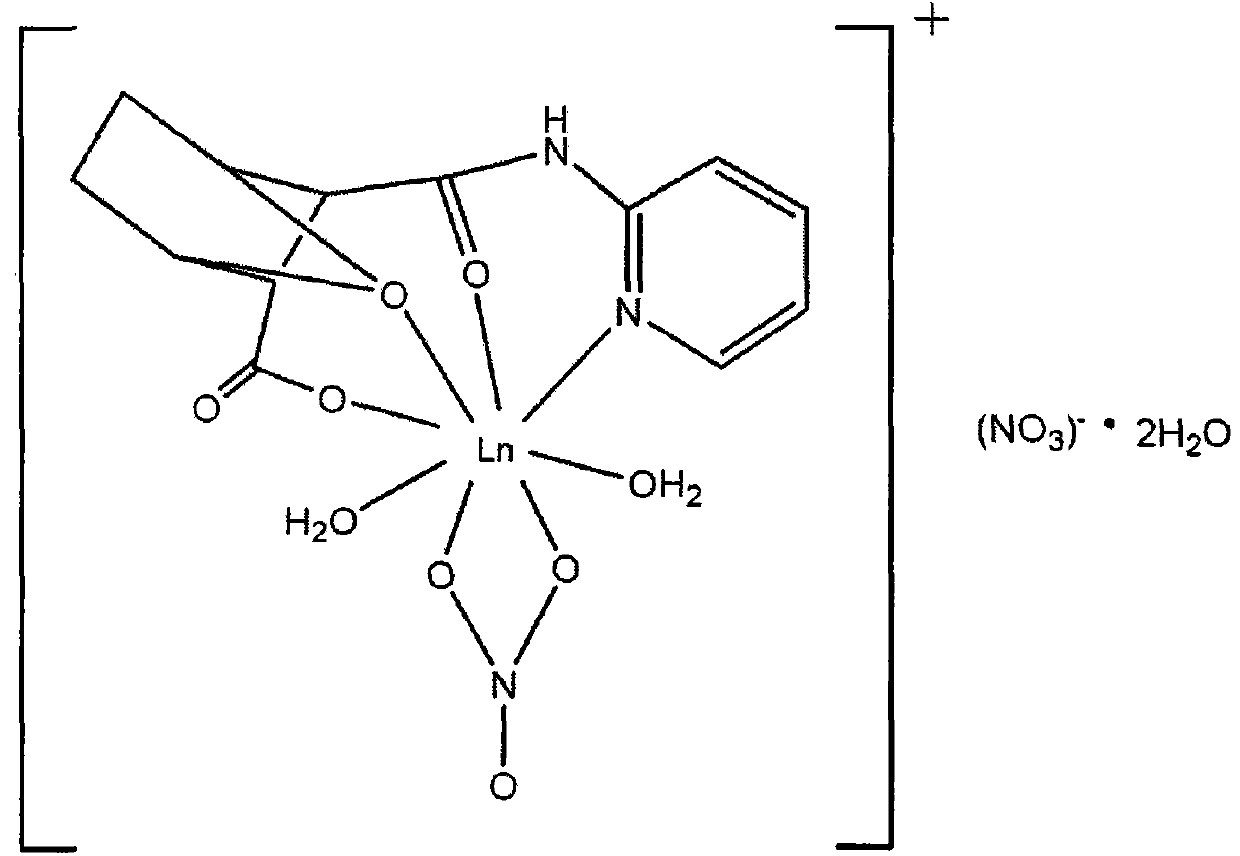
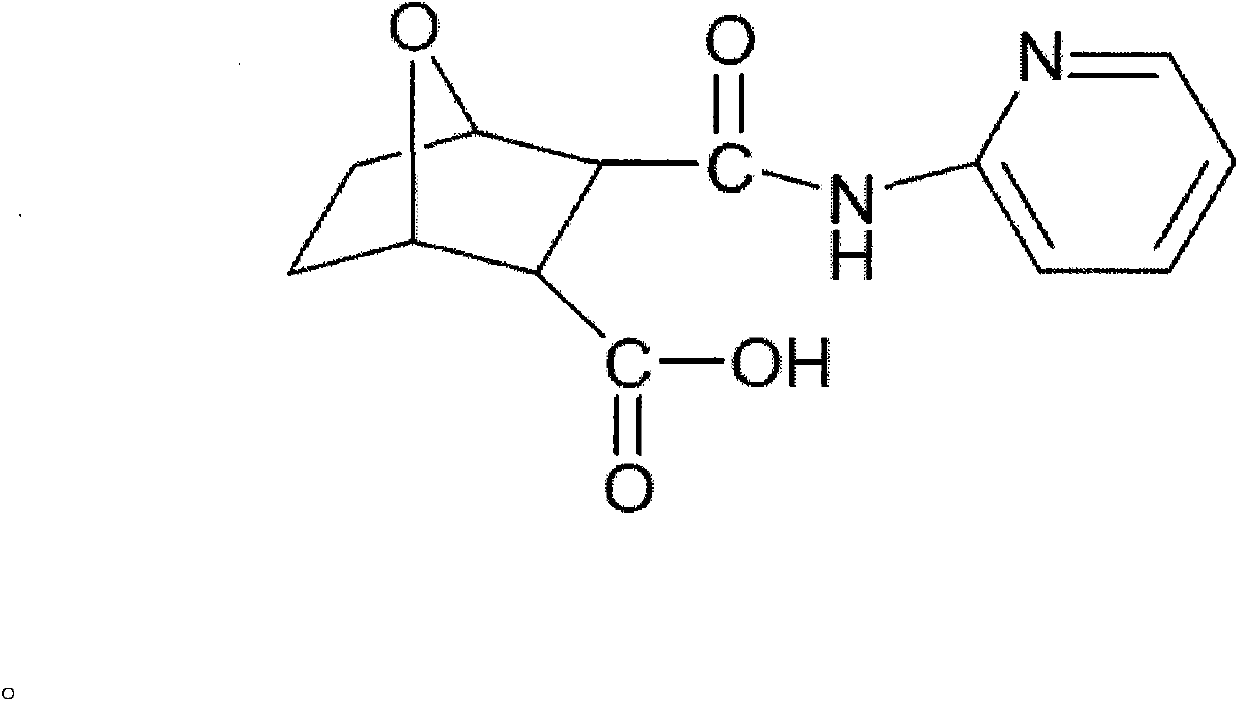
N-pyridine-demethyl cantharidin amic acid lanthanum (III) complex and preparation method thereof
A technology of lanthanum methylcantharidin amidate and methylcantharidin amidate, which is applied in the field of N-pyridine-desmethylcantharidin amidate lanthanum amidate complexes and its preparation, to achieve good selective activity, easy reaction, and low toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
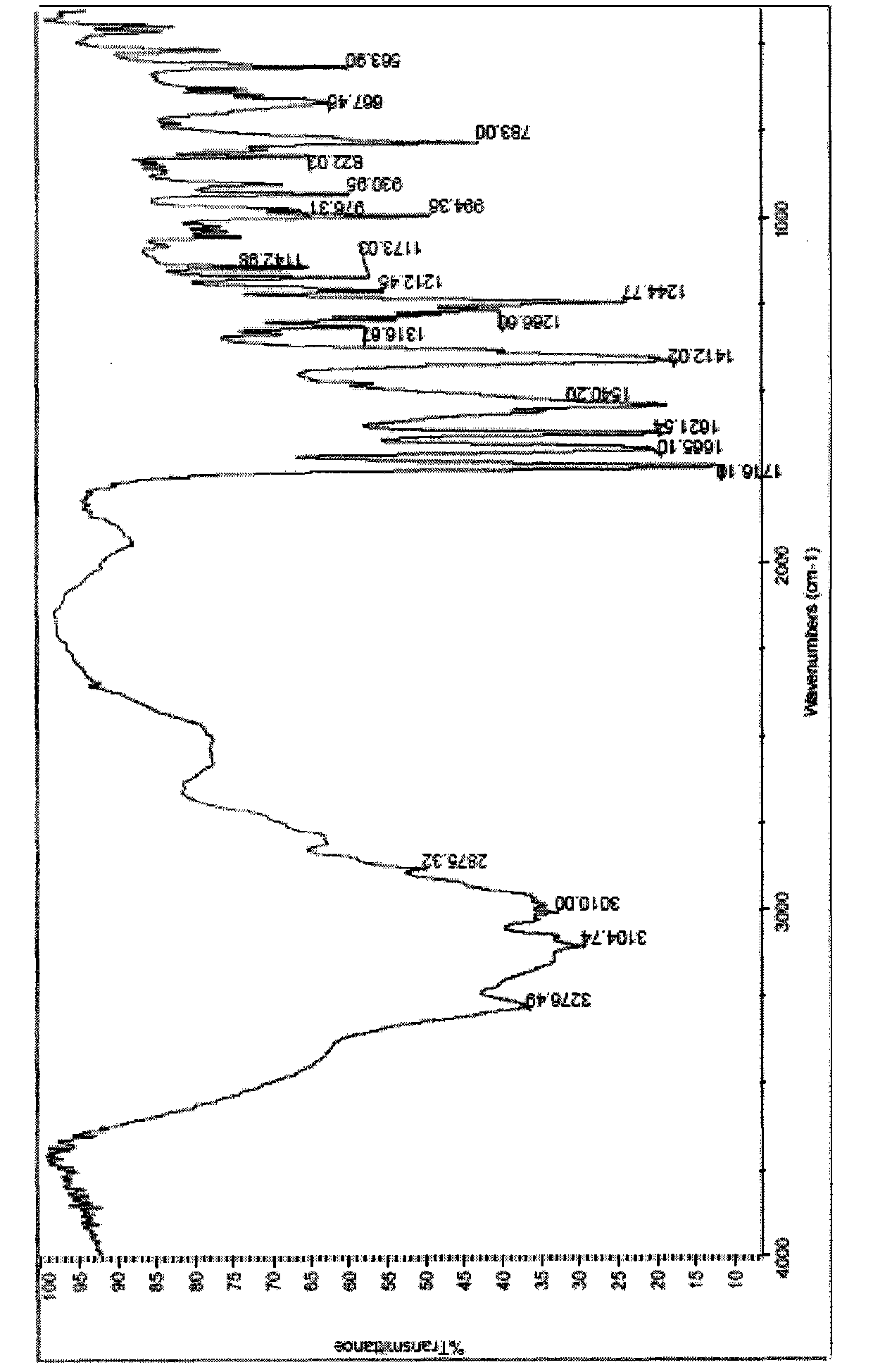
[0029] 1.1 Instrument
[0030] Vario EL III elemental analyzer for elemental analysis
[0031] NEXUS-670 Fourier Transform Infrared Spectrometer for Infrared Spectrum Analysis
[0032] TGA / SDTA851 for thermogravimetric analysis e type thermal analyzer
[0033] UV-2501PC UV-Vis Photometer for UV Spectrum Analysis
[0034] DDS-12 Digital Conductivity Meter for Molar Conductance
[0035] Bruker Avanze 400MHz NMR instrument for proton NMR analysis
[0036] PHS-3C acidity meter
[0037] R201D-II rotary evaporator
[0038] Melting point apparatus: Shanghai Precision Scientific Instrument Co., Ltd. WRS-1B digital melting point apparatus
[0039] 1.2 Reagents
[0040] Demethylcantharidin (Sinopharm Group, chemically pure.); 2-aminopyridine (Sinopharm Chemical Reagent Co., Ltd. Chemically pure); rare earth oxide M 2 o 3 (content > 99.9%, China Pharmaceutical Group Shanghai Chemical Reagent Company); lanthanum La (NO 3 ) 3 ·nH 2 O; acetonitrile; absolute ethanol; methanol; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com