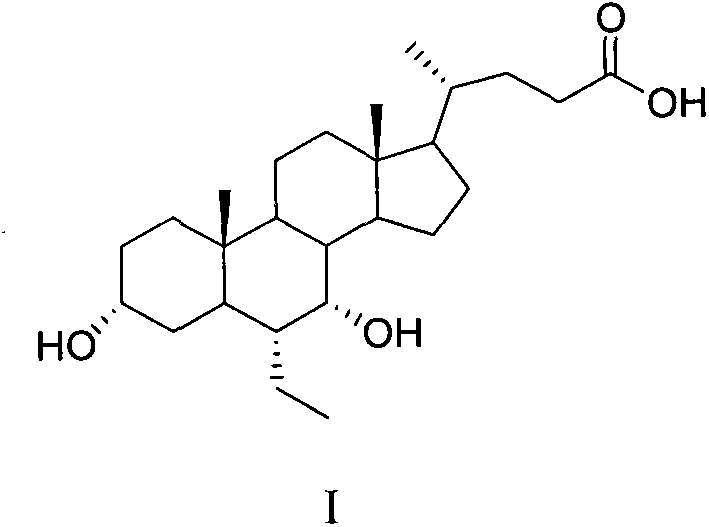
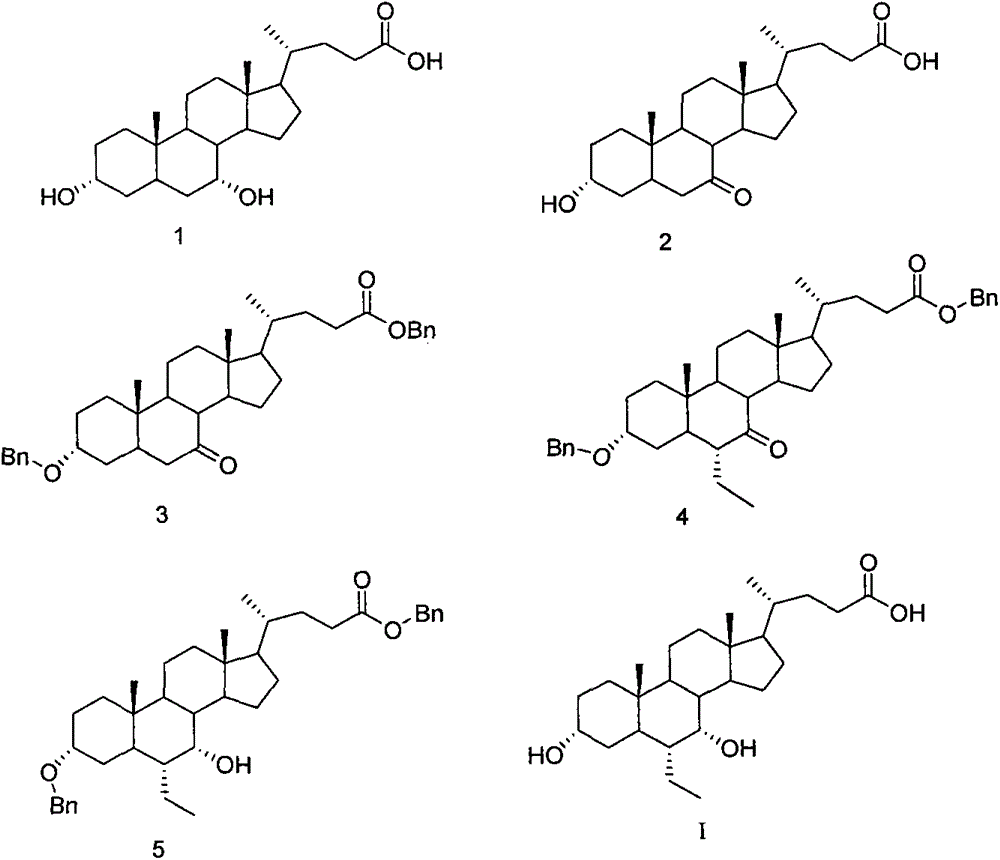
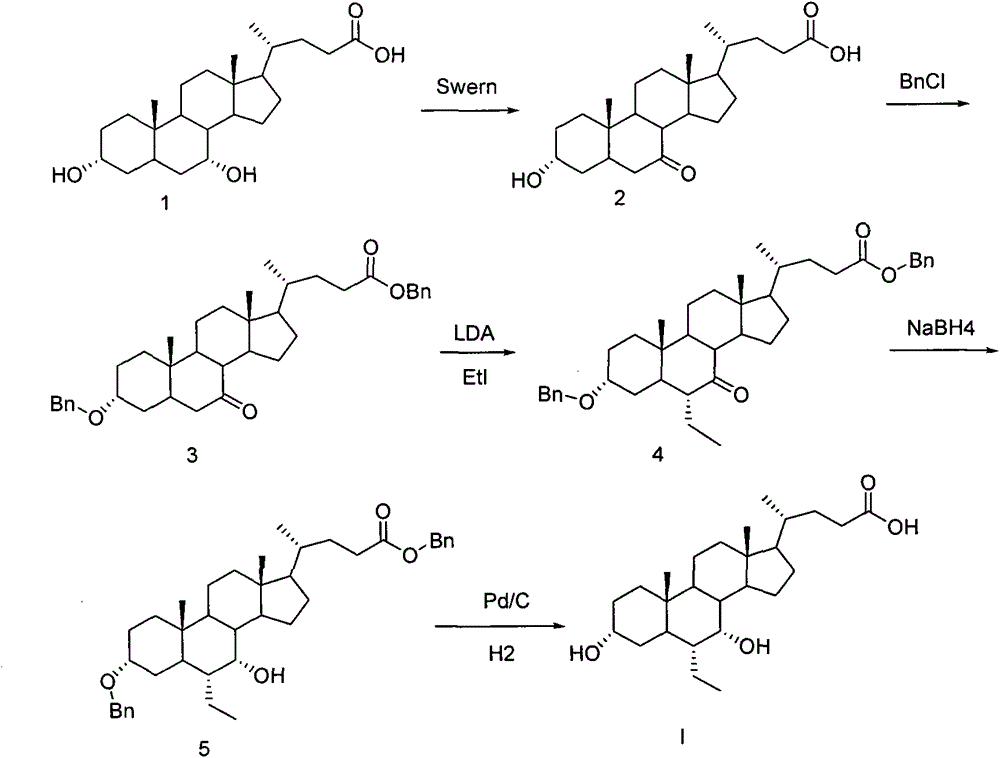
Preparation method of chenodeoxycholic acid derivative
A compound and oxidation reaction technology, applied in the field of preparation of chenodeoxycholic acid derivatives, can solve the problems of low product yield, long process route, 6ECDCA needs to be improved, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Compound 1 (5 g, 12.7 mmol) was added to the reaction flask, and 50 mL of dichloromethane, DMSO (1.12 g, 14.3 mmol), and triethylamine (4.2 g, 41.5 mmol) were added. The reaction temperature was lowered to -30°C, trifluoroacetic anhydride (3.2 g, 15.2 mmol) was added dropwise thereto, and after 1.5 h of heat preservation reaction, 10 mL of water was added, stirred to rise to room temperature, left to separate liquids, and the water phase was dichloromethane Methane was extracted twice, 20ml each time, the organic phases were combined, washed once with saturated sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and the organic phase was concentrated to dryness to obtain compound 2. The yield was 4.89g, and the yield was 98.6%. .
[0039] The analysis data is:
[0040] 1 H NMR (CD3OD) δ3.50(m, 1H), 2.94(m, 1H), 2.52(t, 1H), 2.30(m, 2H), 2.19(m, 6H), 1.70(m, 2H), 1.43 (m, 4H), 1.31(m, 6H), 1.19(s, 3H), 1.12(m, 4H), 0.92(d, 3H), 0.67(s, 3H).
Embodiment 2
[0042] Compound 1 (5 g, 12.7 mmol) was added to the reaction flask, and 50 mL of dichloromethane, DMSO (1.0 g, 12.8 mmol), and triethylamine (3.5 g, 34.6 mmol) were added. The reaction temperature was lowered to -50°C, trifluoroacetic anhydride (2.7g, 12.8mmol) was added dropwise thereto, and after the heat preservation reaction for 0.5h, the post-treatment was the same as in Example 1 to obtain compound 2 with a yield of 4.80g and a yield of 96.8 %.
Embodiment 3
[0044] Compound 1 (5 g, 12.7 mmol) was added into the reaction flask, and 50 mL of dichloromethane, DMSO (3 g, 38.1 mmol), and triethylamine (4.2 g, 41.5 mmol) were added. The reaction temperature was lowered to -70°C, and trifluoroacetic anhydride (8 g, 38.1 mmol) was added dropwise thereto. After 3 hours of heat preservation, the post-treatment was the same as in Example 1 to obtain compound 2 with a yield of 4.76 g and a yield of 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com