Bile duct cancer diagnosis and treatment molecular marker and application thereof
A technology of cholangiocarcinoma and drugs, applied in the biological field, can solve the problems that the early diagnosis of cholangiocarcinoma cannot be applied, and achieve the effect of timely gene diagnosis, lower mortality rate, and better gene diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Screening for gene markers associated with cholangiocarcinoma
[0051] 1.1 Sample collection
[0052] Eight samples of normal bile duct tissue and cholangiocarcinoma tissue were collected. The above samples are surgical resection specimens of patients with cholangiocarcinoma, and all the above samples were obtained with the consent of the organizational ethics committee.
[0053] 1.2 RNA sample preparation and quality analysis
[0054] 1.2.1 RNA sample preparation
[0055] Normal cholangiocarcinoma tissue and cholangiocarcinoma tissue were subjected to RNA extraction to determine the quality of RNA samples by agarose gel electrophoresis, and qualified ones could be used for further transcriptome analysis.
[0056] 1.2.2 Quality analysis of RNA samples (NanoDrop1000 spectrophotometer)
[0057] NanoDrop1000 spectrophotometer detects RNA samples, and the sample requirements for RNA-seq sequencing: OD260 / OD280 is 1.8-2.2.
[0058] 1.2.3 Quality analysis of RN...
Embodiment 2
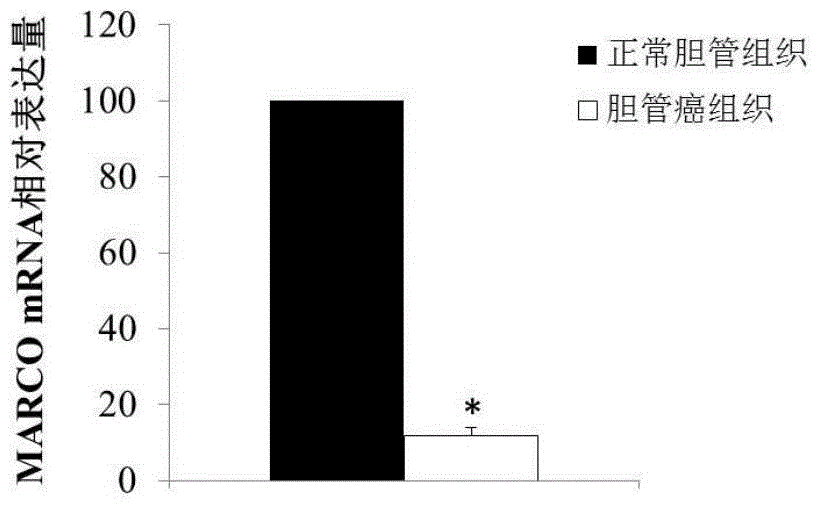
[0069] Example 2 RT-PCR sequencing to verify the differential expression of the MARCO gene
[0070] 1. According to the detection results of high-throughput sequencing, the MARCO gene was selected for large-sample RT-PCR verification. According to the sample collection method in Example 1, 80 cases of cholangiocarcinoma tissue and 80 cases of normal bile duct tissue were selected.
[0071] 2. The RNA extraction process is the same as in Example 1.
[0072] 3. Reverse transcription: TAKARA's reverse transcription kit is used for reverse transcription of RNA, and the specific operation steps are shown in the instruction manual.
[0073] 4. PCR amplification
[0074] The primer sequence of MARCO gene RT-PCR is as follows:
[0075] MARCO: The forward primer is 5'-AGCAAGGAGTAAAGGGAGAA-3' (SEQ ID NO.3);
[0076] MARCO: The reverse primer is 5'-GGTTACTACTGCCGACAATC-3' (SEQ ID NO.4).
[0077] The RT-PCR primer sequence of β-actin gene is as follows:
[0078] β-actin: the forward...
Embodiment 3
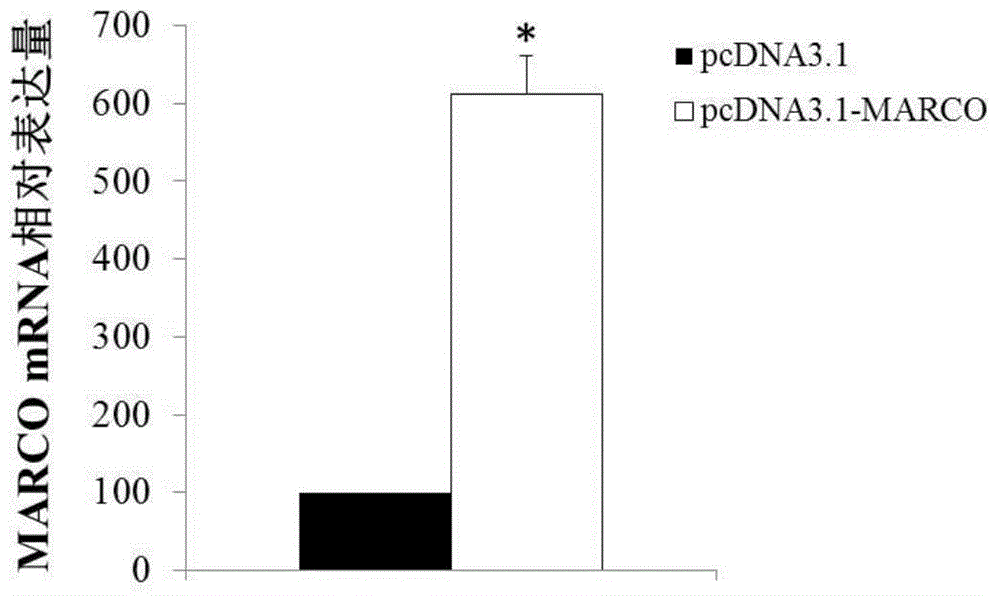
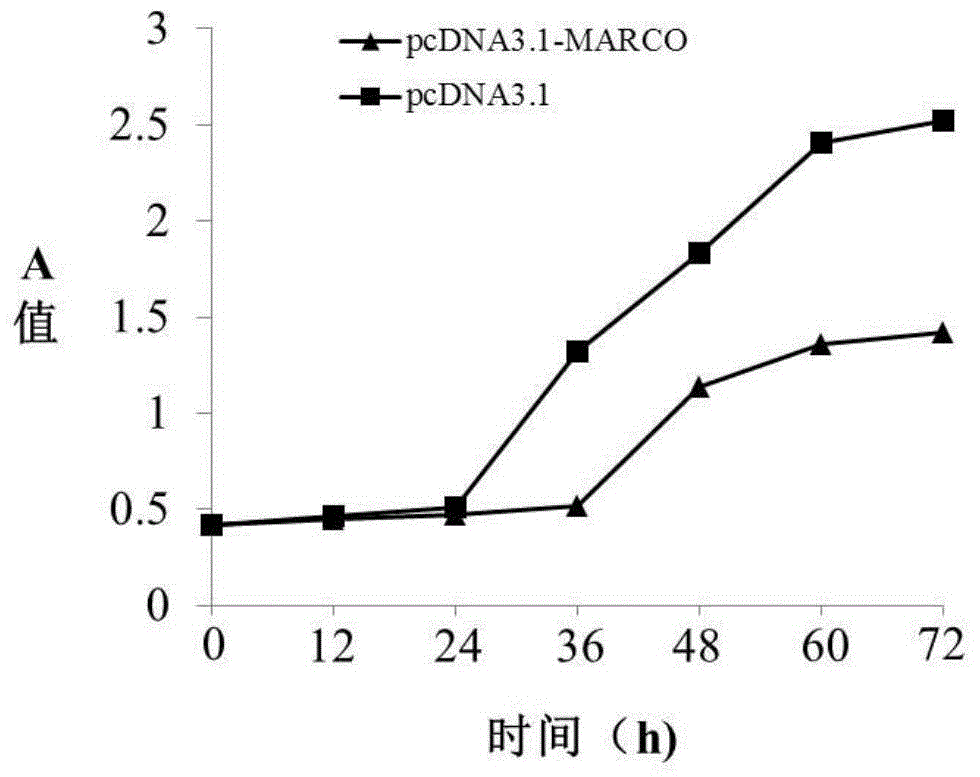
[0090] Example 3 MARCO gene overexpression
[0091] 1. Human cholangiocarcinoma cell line QBC939 was cultured at 37°C and 5% CO in DMEM (high glucose) medium containing 10% calf serum. 2 , Cultivated in an incubator with a relative humidity of 90%. The medium was changed once every 2-3 days, and 0.25% trypsin was used for routine digestion and passage.
[0092] 2. Overexpression of MARCO gene
[0093] 2.1 Construction of MARCO gene expression vector
[0094] Amplification primers were designed according to the coding sequence of the MARCO gene (as shown in SEQ ID NO.1), and the primer sequences were as follows: the forward primer was 5'-ATGCGGCTGCCAGAGCAA-3' (SEQ ID NO.7), and the reverse primer was 5' - CGGAGTATCTGGTTGGCTG-3' (SEQ ID NO. 8). The coding sequence of the full-length MARCO gene was amplified from the cDNA library of adult fetal brain (clontech company, catalog number: 638831). The obtained recombinant vector pcDNA3.1-MARCO was connected to the eukaryotic cell ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com