Application of rhodococcus equi virulence gene VapA recombinant protein
A technology of Rhodococcus equi and recombinant protein, applied in the direction of antibacterial immunoglobulin, antibacterial drugs, bacterial antigen components, etc., can solve the problems of difficult medical diagnosis and treatment product development and application, and achieve the effect of good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Construction and Identification of Prokaryotic Expression Recombinant Plasmid PMAL-VapA
[0041] 1. Resuscitation and cultivation of Rhodococcus equi: Purchase Rhodococcus equi preserved in the American Type Culture Collection with the preservation number ATCC 33701, and recover Rhodococcus equi according to the prescribed operating procedures.
[0042] 2. Primer design: According to the VapA sequence of the Rhodococcus equi pathogenic gene (JN990991.1) in the NCBI gene bank and the pZeroBack / blunt and PMAL-C5x vector restriction sites, use OLigo6.0 software to design a pair of enzyme cutting sites The primers (synthesized by Shanghai Invitage Biotechnology Co., Ltd.), the primer sequences are as follows:
[0043] F: AAGGAAAAAAA GCGGCCGC ATGAAGACCCTGCACAAGACGGTCTC (underlined as not I restriction site)
[0044] R: CCG GAATTC CTAAGCGTTGTGCCAACTACCCGAG (underlined as EcoR I restriction site)
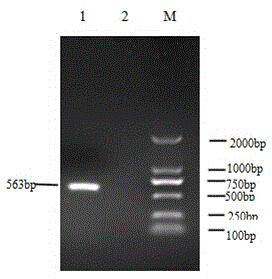
[0045] 3. PCR amplification and cloning of VapA ge...
Embodiment 2
[0088] Example 2 Expression of soluble MBP-VapA recombinant protein and determination of optimal induction conditions
[0089] Inoculate the positive BL21 bacterial liquid transformed with PMAL-VapA plasmid into LB liquid medium (containing ampicillin 50 μg / ml) at a ratio of 1:100, and culture in a shaker at 37°C at 200 r / min. To bacterial solution OD 600 When the value is about 0.6, optimize the expression of MBP-VapA recombinant protein according to the following method.
[0090] 1. Determination of temperature for inducing expression
[0091] IPTG at a concentration of 1 mM was added, and cultured with shaking at 10°C, 15°C, 20°C, 28°C and 37°C, respectively, until the appropriate induction time was taken. The sample was centrifuged at 4000r / min for 10 min, the supernatant was discarded, the precipitate was taken, and the precipitate was resuspended with 1 / 10 of the original volume of deionized water, and the expression of the fusion protein was detected b...
Embodiment 3
[0120] Example 3 Preparation of rabbit hyperimmune serum against Rhodococcus equi VapA
[0121] 1. Antibody preparation
[0122] The immune antigen was selected from the fusion protein MBP-VapA obtained through prokaryotic expression and purification in Example 2 of the present invention.
[0123] Experimental animals: SPF grade male New Zealand white rabbits (1.5-2kg), SPF Kunming mice (18-22g), provided by the Experimental Animal Center of Guangzhou Southern Medical University.
[0124] The preparation method is as follows:
[0125] Basic immunization: Fully emulsify with purified fusion protein MBP-VapA and Montanide Gel 01 ST adjuvant (final concentration ratio: 8%). Rabbits were injected subcutaneously on the back of the neck, and 1.0mL of antigen emulsion (containing about 1mg of antigen) was injected at multiple points, with an average of 0.2mL per point; mice were injected intraperitoneally, with a dose of 200uL of antigen emulsion (containing about 200ug of an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com