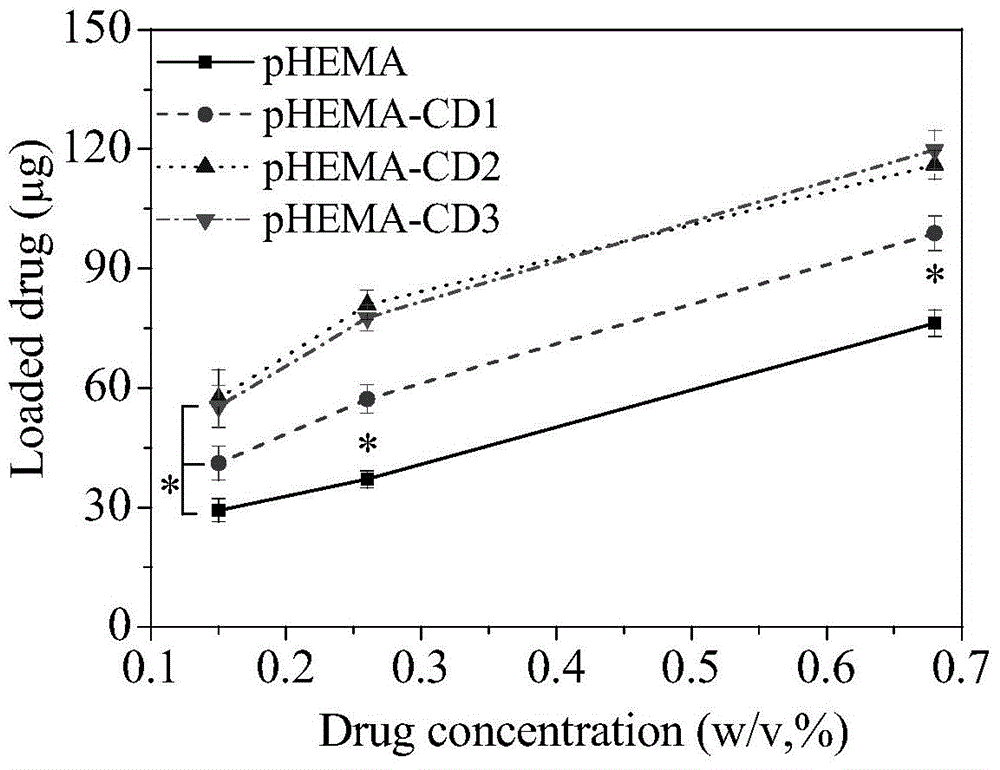
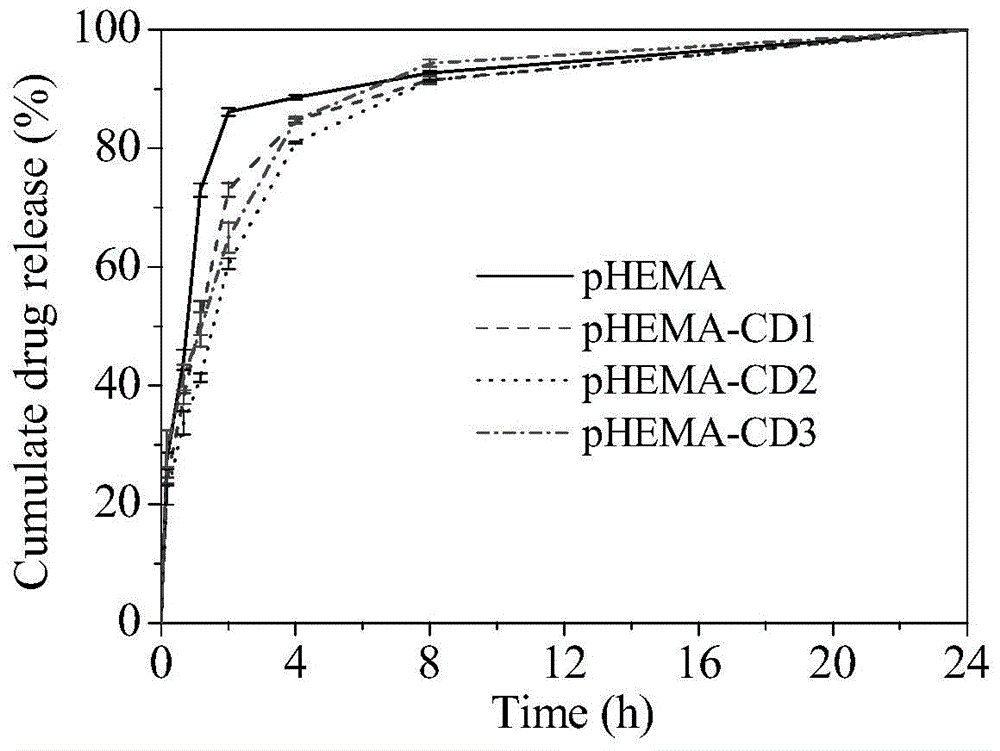
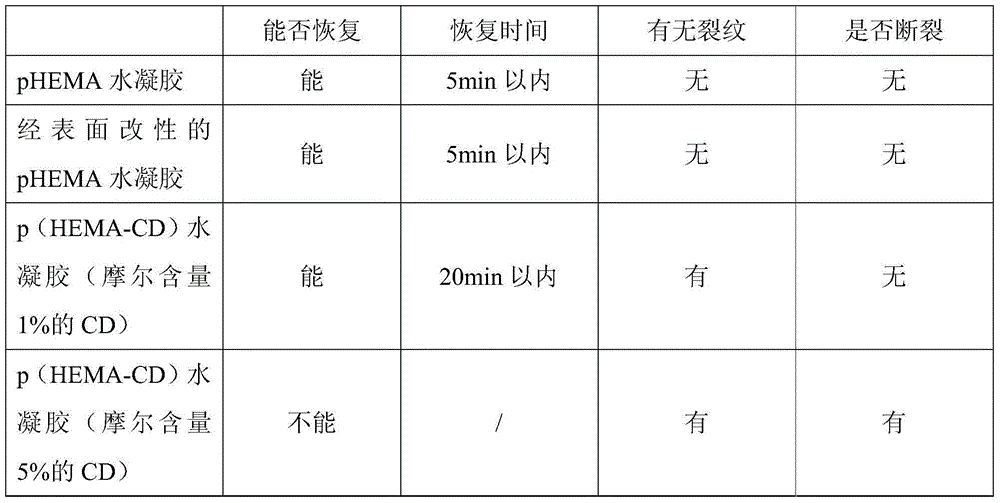
Environment-friendly surface modification method for preparing hydrogel drug carrier
An environmentally friendly and surface modification technology, applied in the direction of inactive components of polymer compounds, can solve problems such as poor mechanical properties and cumbersome methods, and achieve great social and economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 (pHEMA-CD1)
[0034] Put the prepared 0.3g pHEMA hydrogel sheets (diameter 1.5mm, thickness 150μm, 5 pieces) into the reaction vessel, add 10mL acetone, then add 650μL propyne bromide, then add 0.96g potassium carbonate as a catalyst, seal After 2 hours of magnetic stirring reaction, the hydrogel sheet was taken out and put into water to terminate the reaction, and the unreacted monomer and catalyst were washed away with water and acetone to obtain an alkynylated hydrogel sheet. The grafting rate of alkynyl groups on the hydrogel was determined to be 10.13% by mass method.
[0035] Dissolve 268 mg of mono(6-mercapto-6-deoxy)-β-cyclodextrin (purchased directly) in 20 mL of a mixed solvent of tetrahydrofuran and water at a ratio of 1:1, and then dissolve 0.3 g of alkynylated The hydrogel sheet is placed in a solution containing cyclodextrin, and finally photoinitiator I2959 is added at a concentration of 0.1%. After stirring evenly, it is sealed, and then react...
Embodiment 2
[0036] Example 2 (pHEMA-CD2)
[0037] Put the prepared 0.3g pHEMA hydrogel sheets (diameter 1.5mm, thickness 150μm, 5 pieces) into the reaction vessel, add 10mL acetone, then add 650μL propyne bromide, then add 0.96g potassium carbonate as a catalyst, seal After 2 hours of magnetic stirring reaction, the hydrogel sheet was taken out and put into water to terminate the reaction, and the unreacted monomer and catalyst were washed away with water and acetone to obtain an alkynylated hydrogel sheet. The grafting rate of alkynyl groups on the hydrogel was determined to be 10.13% by mass method.
[0038] Dissolve 538 mg of mono(6-mercapto-6-deoxy)-β-cyclodextrin (purchased directly) in 20 mL of a mixed solvent of tetrahydrofuran and water at a ratio of 1:1, and then 0.3 g of the above alkyne The hydrogel sheet is placed in a solution containing cyclodextrin, and finally photoinitiator I2959 is added at a concentration of 0.1%. After stirring evenly, it is sealed, and then reacted u...
Embodiment 3
[0039] Example 3 (pHEMA-CD3)
[0040] Put the prepared 0.3g pHEMA hydrogel sheets (diameter 1.5mm, thickness 150μm, 5 pieces) into the reaction vessel, add 10mL acetone, then add 650μL propyne bromide, then add 0.96g potassium carbonate as a catalyst, seal After 2 hours of magnetic stirring reaction, the hydrogel sheet was taken out and put into water to terminate the reaction, and the unreacted monomer and catalyst were washed away with water and acetone to obtain an alkynylated hydrogel sheet. The grafting rate of alkynyl groups on the hydrogel was determined to be 10.13% by mass method.
[0041] Dissolve 1.34 g of mono(6-mercapto-6-deoxy)-β-cyclodextrin (purchased directly) in 20 mL of a mixed solvent of tetrahydrofuran and water at a ratio of 1:1, and then add the above 0.3 g of alkynyl Place the degraded hydrogel sheet in a cyclodextrin-containing solution, and finally add photoinitiator I2959 at a concentration of 0.1%, stir evenly and seal it, and react under sunlight ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com