Preparation method of diphenhydramine
A technology for diphenhydramine and benzhydryl alcohol, which is applied in the field of preparation of diphenhydramine, can solve the problems that toluenesulfonic acid cannot be recycled and the production cost is high, and achieves easy industrial production, simple operation and high product yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
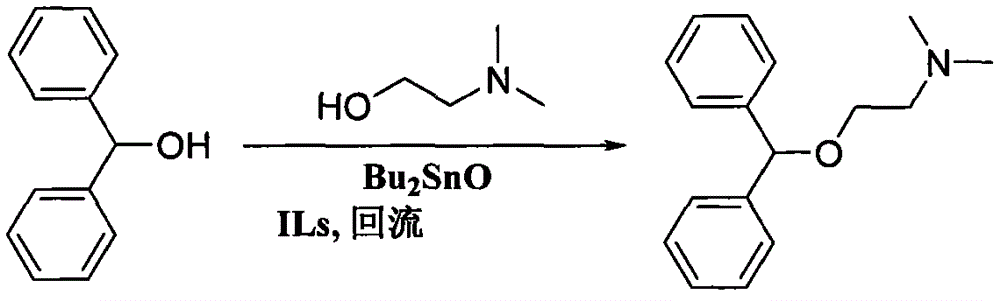
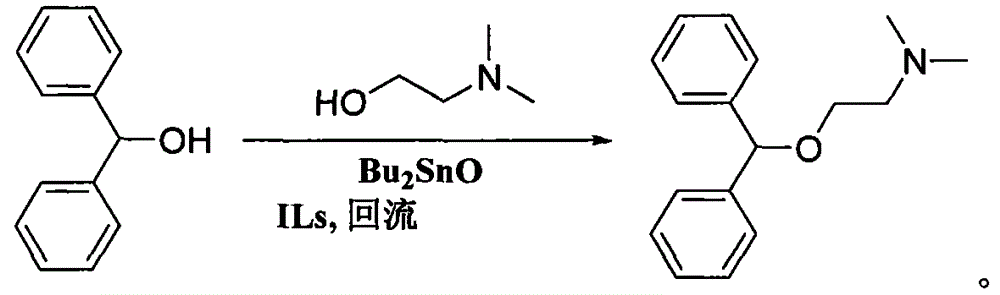
[0024] Add 36.8 grams (0.2mol) of diphenylmethanol, 26.7 grams (0.3mol) of dimethylaminoethanol, 2.9 grams (accounting for 8% of the mass of diphenylmethanol) of dibutyltin oxide and ionic liquid N bromide in a 250mL one-mouth bottle - 1.1 grams of n-propylpyridine (accounting for 3% of the mass of diphenylmethanol), the reaction mixture was stirred and reacted under reflux. The progress of the reaction was detected by gas chromatography. After reacting for 10 hours, the reaction was completed, and the excess dimethylaminoethanol (which can be recycled) was removed by distillation under reduced pressure, and then the temperature was raised to carry out distillation under reduced pressure to obtain 49.0 grams of diphenhydramine, with a yield of 96.0%, gas phase analysis 99.5% purity.
Embodiment 2
[0026] Add 36.8 grams (0.2mol) of diphenylmethanol, 26.7 grams (0.3mol) of dimethylaminoethanol, 1.1 grams (accounting for 3% of the mass of diphenylmethanol) of dibutyltin oxide and ionic liquid N bromide in a 250mL one-mouth bottle - 1.1 grams of n-propylpyridine (accounting for 3% of the mass of diphenylmethanol), the reaction mixture was stirred and reacted under reflux. The progress of the reaction was detected by gas chromatography. After reacting for 20h, the reaction was completed, and the excess dimethylaminoethanol (which can be recycled) was removed by distillation under reduced pressure, and then the temperature was increased to carry out distillation under reduced pressure to obtain 48.6 grams of diphenhydramine, with a yield of 95.1%, gas phase analysis 99.0% purity.
Embodiment 3
[0028] Add 36.8 grams (0.2mol) of diphenylmethanol, 35.6 grams (0.4mol) of dimethylaminoethanol, 3.68 grams (accounting for 10% of the mass of diphenylmethanol) of dibutyltin oxide and ionic liquid bromide 1 in a 250mL one-port bottle. - 1.8 g of methyl-3-ethylimidazolium salt (accounting for 5% of the mass of benzhydryl alcohol), and the reaction mixture was stirred and refluxed for reaction. The progress of the reaction was detected by gas chromatography. After reacting for 8 hours, the reaction was completed, and the excess dimethylaminoethanol (which can be recycled) was removed by distillation under reduced pressure, and then the temperature was raised to carry out distillation under reduced pressure to obtain 49.5 grams of diphenhydramine, with a yield of 97.0%, gas phase analysis 99.6% purity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com