Novel substituted aminophenol compounds and preparation method and application thereof in field of medicine
An aminophenol and compound technology, applied in the field of substituted aminophenol compounds and their preparation, achieves the effects of significant anti-breast cancer activity, high yield and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
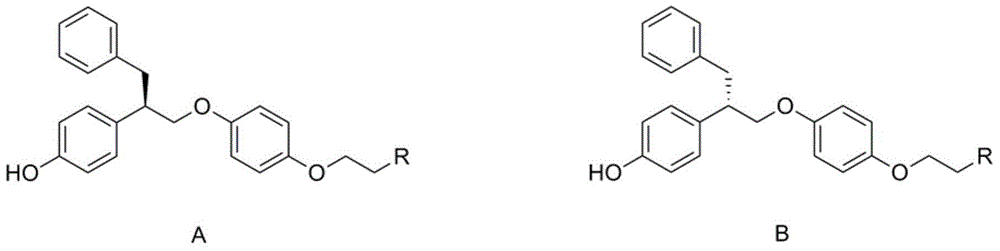
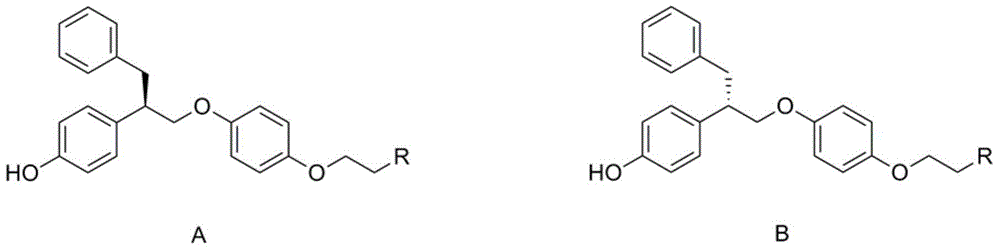
[0037] The present invention provides a substituted aminophenol compound with good anti-breast cancer activity, its preparation method and application, and its structure is shown in general formula A or B:
[0038]
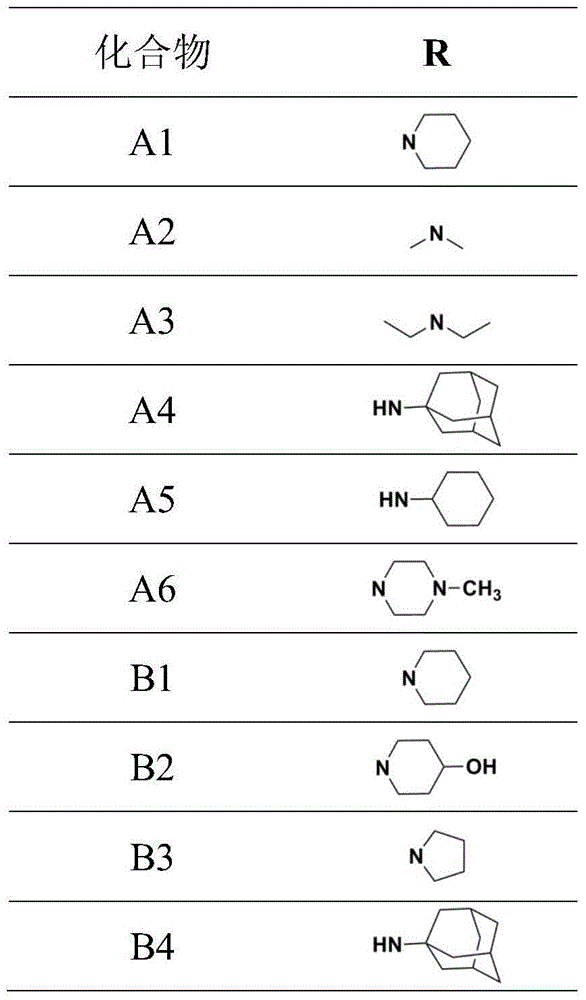
[0039] Wherein, R is selected from a or b, and is connected with an ethoxy group through an amino group.
[0040] a.R is a secondary amine, (a) 1 to 6 carbon atom chain alkyl secondary amines, wherein the alkyl group can be straight or branched; (b) cycloalkyl secondary amines, wherein the cycloalkyl group includes cyclopentyl group, cyclohexyl, cycloheptyl and adamantyl;
[0041] b. R is a tertiary amine, (a) a chained alkyl tertiary amine with 1 to 6 carbon atoms, wherein the alkyl group can be a straight chain or a branched chain, and the two alkyl groups of the tertiary amine can be the same or different; ( b) alicyclic amines, including pyrrole, piperidine, piperazine and morpholine; (c) substituted piperidines and substituted piperazines, wherein the sub...
Embodiment 1
[0072] Embodiment 1 prepares intermediate 5
[0073] 1. Preparation of (2-(4-benzyloxy)phenoxy)ethanol
[0074]
[0075] Take 10.00g (0.05mol) of 4-benzyloxyphenol into a three-necked flask, add 50mL of water, heat to 80°C, add 63mL of 6% sodium hydroxide aqueous solution dropwise to a constant pressure dropping funnel under mechanical stirring, and the solution gradually becomes clear , 30 minutes to drop. Dissolve 12.5 g (0.10 mol) of bromoethanol in 20 mL of water, add dropwise to the above reaction solution through a constant-pressure dropping funnel, react at 80°C for 5 hours, adjust the pH to acidic, a white solid precipitates, extract with dichloromethane three times, wash with water twice Once, dried with saturated saline, and evaporated to dryness, recrystallized from dichloromethane and petroleum ether to obtain about 11.96 g of white crystals, with a yield of 98%. 1 H NMR (300MHz, CDCl 3 )δ3.91-3.94(m,2H),4.01-4.04(m,2H),5.01(s,2H),6.83-7.44(m,9H).
[0076] 2...
Embodiment 2
[0085] Embodiment 2 prepares intermediate 8
[0086] 1. Preparation of methyl 4-benzyloxyphenylacetate
[0087]
[0088] Weigh 20.00g (120.48mmol) of methyl p-hydroxyphenylacetate and dissolve it in 400mL of acetone, add 33.25g (240.96mmol) of potassium carbonate, then measure 16mL (132.53mmol) of benzyl bromide and add to the above reaction solution in batches. Stir at room temperature and react overnight. After the reaction, the solvent was evaporated to dryness, the solid was dissolved in dichloromethane, washed twice with water and once with saturated brine, and dried over anhydrous sodium sulfate. After filtering and evaporating the solvent to dryness, about 30.84 g of a colorless oil was obtained, with a yield of 97%. 1H NMR (300MHz, CDCl 3 )δ3.58(s,2H),3.69(s,3H),5.06(s,2H),6.93-7.45(m,9H).
[0089] 2. Preparation of 4-benzyloxyphenylacetic acid
[0090]
[0091] Take 30.84g (0.12mol) of methyl 4-benzyloxyphenylacetate and dissolve it in 100mL of methanol, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com