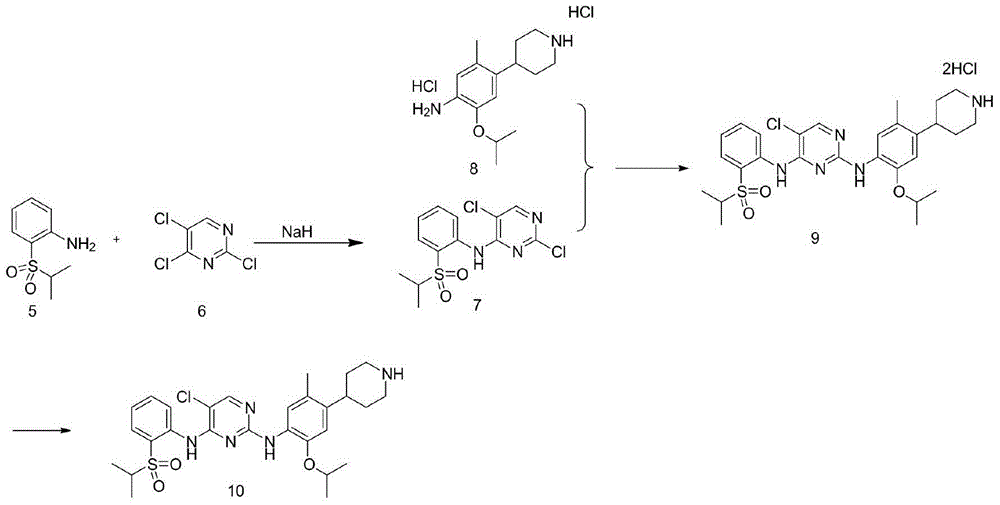
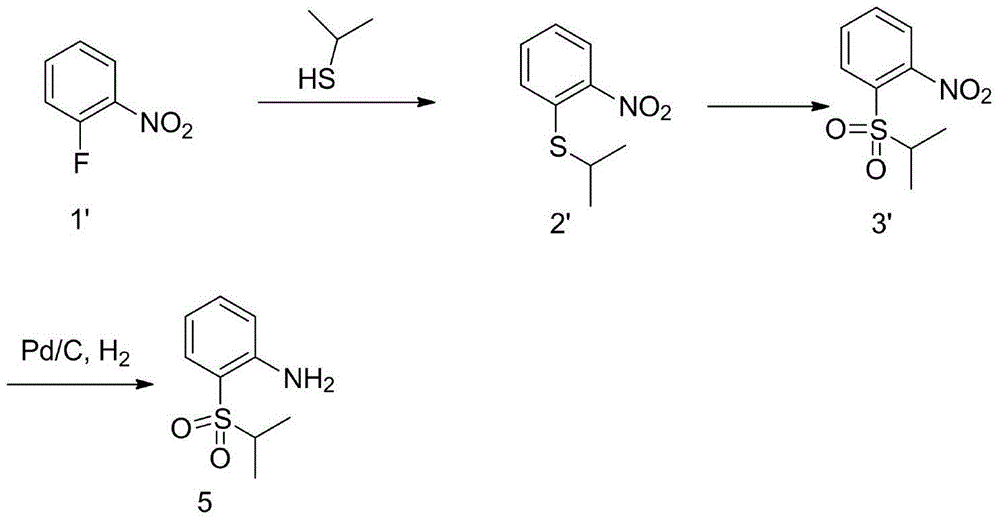
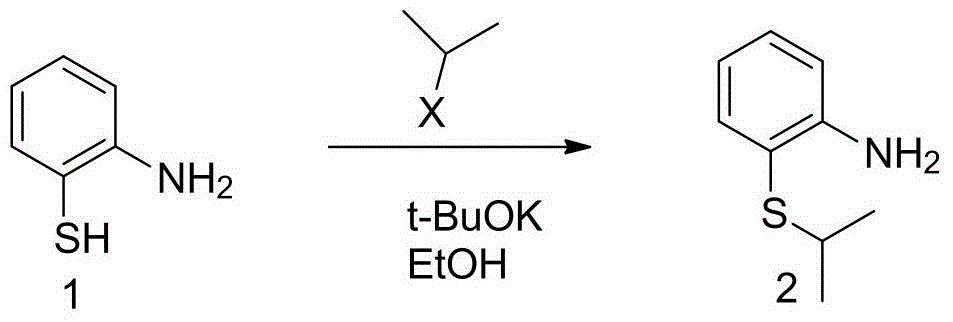Preparation method of 2,5-dichloro-N-(2-isopropylsulfonyl) phenyl) pyrimidine-4-amine
A technology of propylsulfonyl and benzenesulfonyl, which is applied in the field of preparation of ceritinib to achieve the effects of reduced production cost, stable quality and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment one 2-(isopropyl mercapto) aniline
[0043]
[0044] Add o-aminothiophenol (99.00g) and water (300mL) into the reaction flask, and stir. NaOH aqueous solution. 116.85 g of 2-bromopropane was added dropwise. After the dropwise addition, the temperature was raised to 40-50° C. for 2 hours to react. After the reaction was completed, it was cooled, and ethyl acetate was added to extract twice. The organic phases were combined and washed once with water. The solvent was distilled off to obtain 125.6 g of 2-(isopropylmercapto)aniline. Yield 95%.
[0045] MS (ESI+): 168.1 (M+1)+.
Embodiment 2
[0046] Example two N-(2-(isopropylthio)phenyl)acetamide
[0047]
[0048] 2-(Isopropylmercapto)aniline (132g), acetic acid (132mL) were added into the reaction flask. Stir and heat up to 40°C. Acetic anhydride (96.80 g) was added dropwise. Stir for 30 minutes after the dropwise addition is complete. After the reaction was completed, it was poured into ice water and extracted twice with ethyl acetate. The organic phases were combined and washed once with water. The solvent was distilled off to obtain 161.9 g of N-(2-(isopropylthio)phenyl)acetamide. Yield 98%.
[0049] MS(ESI+):210.1(M+1)+. 1H NMR(CDCl3):δ8.40(d,4.0,1H),7.47-7.49(m,1H),7.30-7.34(m,1H),7.00-7.04(m,1H),3.09-3.14(m, 1H), 2.21(s, 3H), 1.28-1.32(d, 2.6, 6H).
Embodiment 3
[0050] Example three N-(2-(isopropylsulfonyl)phenyl)acetamide
[0051]
[0052] N-(2-(isopropylthio)phenyl)acetamide (165 g), acetic acid (330 mL) were added to the reaction flask. Stir and heat up to 40°C. Sodium perborate tetrahydrate (364 g) was added in portions. Stirring was continued at 40°C for 2 hours after the addition was complete. Raise the temperature to 50-60°C and continue the reaction until the reaction is complete. Cool to 20-30°C. Add to ice water. Suction filtration, the filter cake was washed twice with water. Collect the filter cake. After drying, 173 g of N-(2-(isopropylsulfonyl)phenyl)acetamide was obtained as a tan solid. Yield 91%
[0053] MS (ESI+): 242.1 (M+1)+. 1H NMR(CDCl3):δ8.46-8.50(m,1H),7.79-7.83(m,1H),7.59-7.65(m,1H),7.20-7.25(m,1H),3.16-3.22(m, 1H), 1.28-1.32 (m, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com