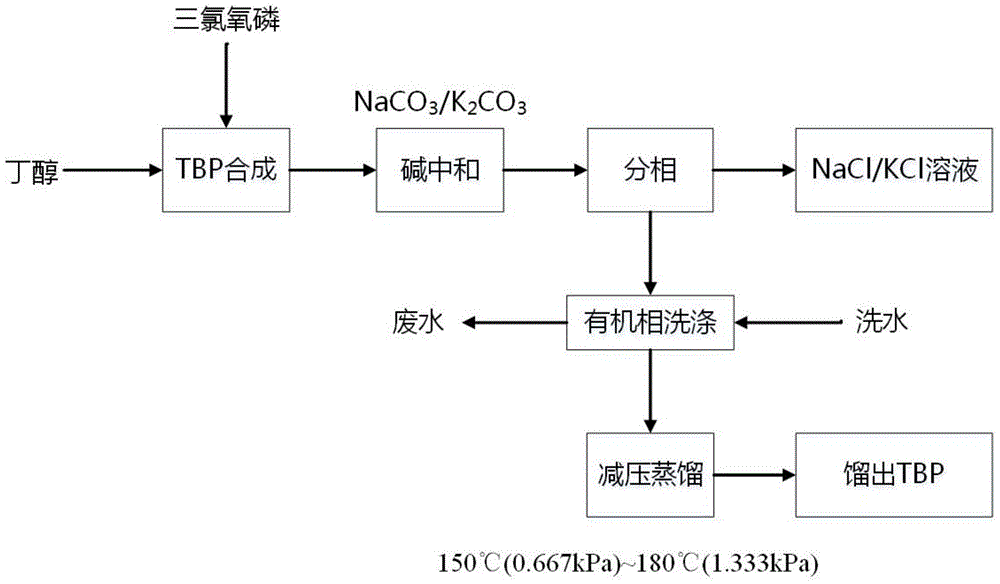
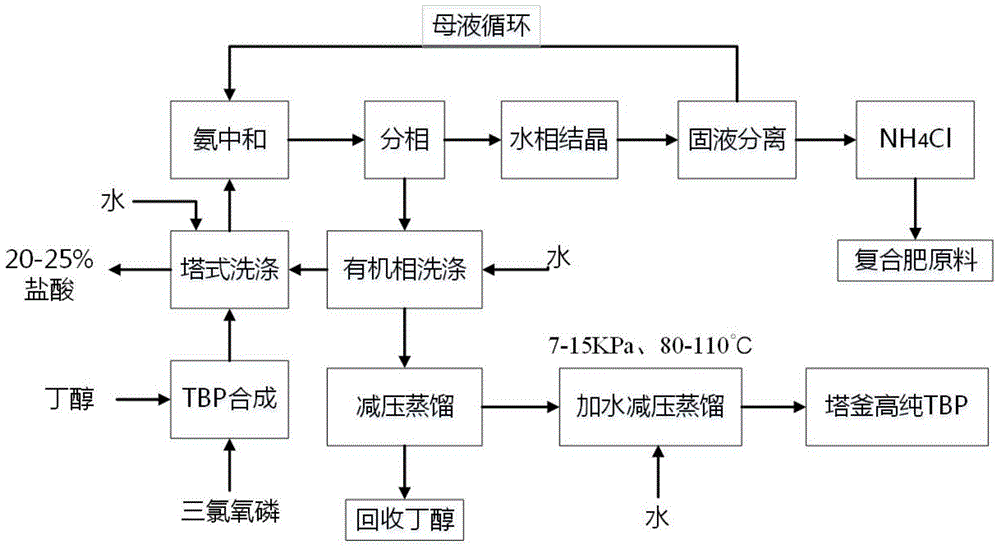
A kind of preparation method of high-purity tributyl phosphate
A technology of high-purity tributyl phosphate and pure tributyl phosphate, applied in the field of purification, can solve the problems of low purity, inability to recycle, environmental pollution, etc., and achieve the effects of reducing the amount of alkali, mild conditions, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 296.48g (i.e. 4mol) of n-butanol into the esterification kettle, turn on the frozen brine to keep the temperature in the kettle below 10°C, add 76.67g (i.e. 0.5mol) of phosphorus oxychloride dropwise under stirring, and control the reaction temperature at 20°C. ℃-25℃, stirring until the reaction is completed. Add 150 ml of water to the reacted mixture and wash it with a multi-stage water washing tower, and let it stand to separate into layers. The aqueous phase yields a 24.7% hydrochloric acid solution. The organic phase was neutralized to pH 7 with ammonia gas. Ammonium chloride was crystallized from the neutralized aqueous phase, and then solid-liquid separation was performed to obtain 8.475 g of ammonium chloride. The mother liquor returns to the neutralization stage for circulation.
[0029] The organic phase was washed with 200ml of water and the pH was controlled to be 6-7, and the crude dealcoholization was carried out by distillation under the conditions ...
Embodiment 2
[0031] Add 296.48g (i.e. 4mol) of n-butanol into the esterification kettle, turn on the frozen brine to keep the temperature in the kettle below 10°C, add 76.67g (i.e. 0.5mol) of phosphorus oxychloride dropwise under stirring, and control the reaction temperature at 20°C. ℃-25℃, stirring until the reaction is completed. Add 180 ml of water to the reacted mixture and wash it with a multi-stage water washing tower, and let it stand to separate layers. The aqueous phase yields a 27.5% hydrochloric acid solution. The organic phase was neutralized to pH 7 with ammonia gas. Ammonium chloride was crystallized from the neutralized aqueous phase, and then solid-liquid separation was performed to obtain 8.475 g of ammonium chloride. The mother liquor returns to the neutralization stage for circulation.
[0032] The organic phase was washed with 200ml of water and the pH was controlled to be 6-7, and the crude dealcoholization was carried out by distillation under the conditions of 15...
Embodiment 3
[0034] Add 296.48g (i.e. 4mol) of n-butanol into the esterification kettle, turn on the frozen brine to keep the temperature in the kettle below 10°C, add 76.67g (i.e. 0.5mol) of phosphorus oxychloride dropwise under stirring, and control the reaction temperature at 20°C. ℃-25℃, stirring until the reaction is completed. Add 160ml of water to the reacted mixture and wash it with a multi-stage water washing tower, and let it stand to separate layers. The aqueous phase yields a 24.7% hydrochloric acid solution. The organic phase was neutralized to pH 7 with ammonia gas. Ammonium chloride was crystallized from the neutralized aqueous phase, and then solid-liquid separation was performed to obtain 1.695 g of ammonium chloride. The mother liquor returns to the neutralization stage for circulation.
[0035] The organic phase was washed with 200ml of water and the pH was controlled to be 6-7, and the crude dealcoholization was carried out by distillation under the conditions of 15k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com