Pertussis PRN protein monoclonal antibody and application thereof
A monoclonal antibody and pertussis technology, applied in the direction of antibodies, anti-bacterial immunoglobulins, anti-bacterial drugs, etc., can solve the problems such as the loss of efficacy of pertussis vaccines, and achieve high specificity, high sensitivity and accurate detection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, establishment of hybridoma cell line
[0029] 1. Experimental materials
[0030] 1. Immunogen: The PRN protein in pertussis vaccine (purchased from Hualan Bioengineering Co., Ltd.) was used as the immunogen.
[0031] 2. Medium: DMEM medium was purchased from Hyclone Company; HAT, HT selective medium, and pristane were purchased from Sigma Company.
[0032] 3. Experimental animals: Balb / c mice, 8-12 weeks old, female, SPF grade animal culture.
[0033] 4. Other materials: Freund's complete adjuvant and Freund's incomplete adjuvant were purchased from Sigma Company; PEG4000 was purchased from Fluka Company; HRP-goat anti-mouse IgG antibody was purchased from JacksonImmune Company; other reagents were domestic analytically pure products.
[0034] 2. Establishment of hybridoma cell lines
[0035] 1. Animal immunity
[0036] 1) Basic immunization: The immunogen was mixed with Freund's complete adjuvant in equal volumes and fully emulsified, and injected sub...
Embodiment 2
[0050] The preparation of the monoclonal antibody of embodiment 2 anti-PRN protein
[0051] Primary antibody preparation
[0052] Adult BALB / c mice were selected, and pristane was inoculated intraperitoneally, 0.5ml per mouse. After 7-10 days, the 16th generation cells of the two strains of hybridoma cells in Example 1 were inoculated into the peritoneal cavity respectively, and each mouse was 1×10 6 -2×10 6 indivual. After an interval of 5 days, when the abdomen is obviously enlarged and the skin feels tense when touched with hands, the ascites can be collected with a No. 9 needle.
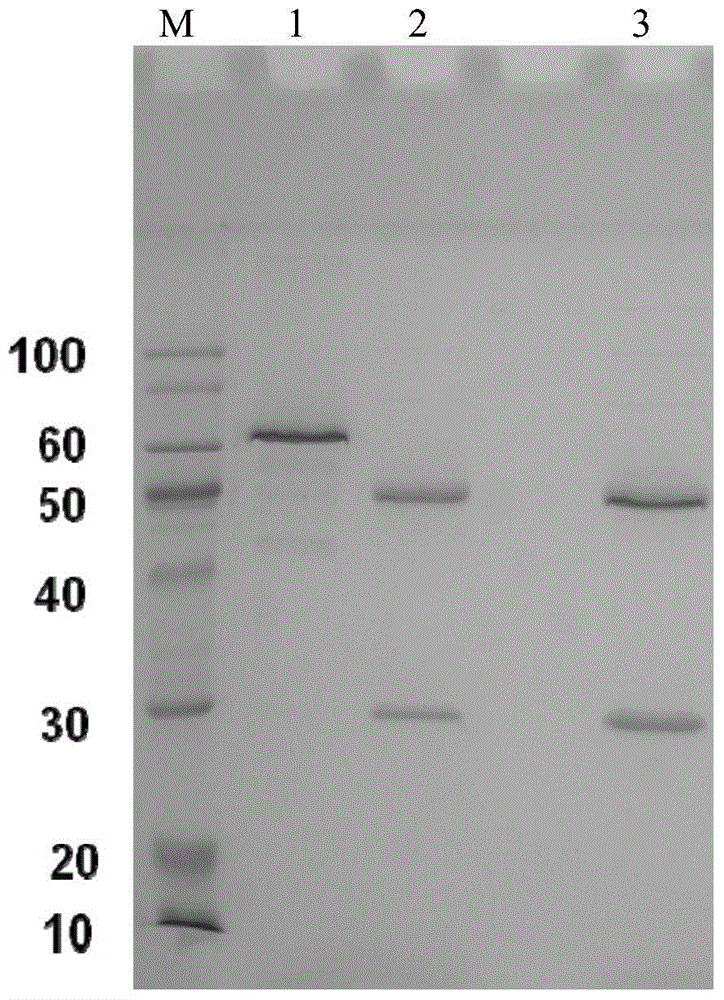
[0053] Centrifuge the ascitic fluid (13000r / min for 30 minutes), remove cell components and other precipitates, and collect the supernatant. Purify with Protein G~Sepharose CL-4B, the upper column solution is 20mM PBS buffer solution, and the column chromatography eluent is: pH2.7, 20mM glycine buffer solution to obtain monoclonal antibodies against PRN protein respectively, wherein The mono...
Embodiment 3
[0059] Example 3 Preparation of Anti-Pertussis PRN Protein Detection Reagent Using Purified Antibody
[0060] 1. Anti-PRN protein ELISA double antibody sandwich method
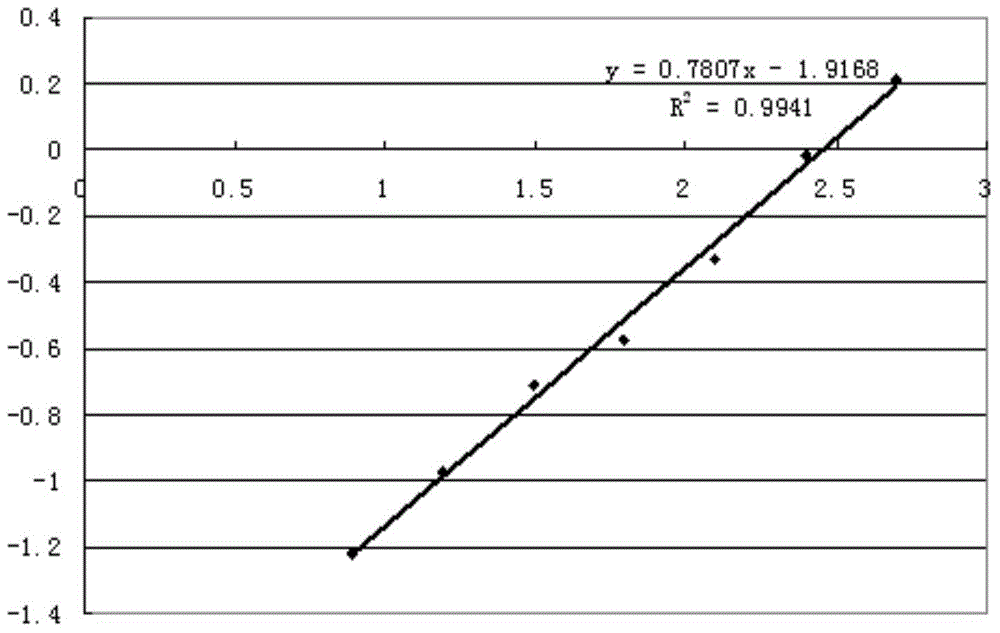
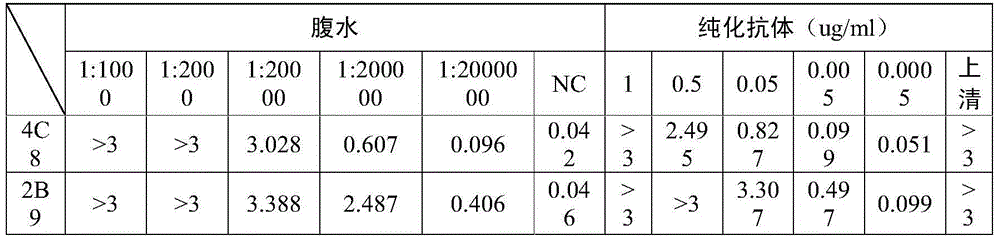
[0061] Using 4C8 and 2B9 antibodies for pairing experiments, it was determined that 4C8 was used as the coating antibody, and HRP-labeled 2B9 was used as the detection antibody. The ELISA detection method was determined, and the detection sensitivity of the kit could reach 7.8125ng / ml ( figure 2 ). Antibodies were labeled with the modified sodium periodate method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com