Anti-TNFalpha fully human monoclonal antibodies and application thereof
A fully human antibody and antibody technology, applied in the fields of application, antibody, anti-animal/human immunoglobulin, etc., can solve the problem of infeasible immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1: Preparation of hybridoma and vector
[0071] 1. Antigen and lymphocyte preparation and immunization
[0072] 1.1 Antigen TNFα protein preparation
[0073] Human TNFα protein (drug target) and monkey TNFα (drug target) protein were purchased from Baiyiqiao Shenzhou, diluted with PBS, filtered with a 0.22μm needle-type sterile filter, and packed into 1.5ml cell cryopreservation tubes in- Store at 80°C.
[0074] 1.2 Human lymphocyte preparation
[0075] Aseptically harvest fresh tissue samples of chronic tonsillitis (in cooperation with the Department of Otolaryngology, Affiliated Hospital of Xuzhou Medical College, tonsils were taken from patients undergoing tonsillectomy, 10 patients, aged 5 to 10 years), and lymphocyte separator (GE) Isolate the lymphocytes and take 30×10 6 A; aseptically extract human fresh peripheral blood cells, separate the lymphocytes with lymphocyte separation solution (GE), and take 30×10 6 One.
[0076] 1.3 In vitro lymphocyte immunity
[0077...
Embodiment 2
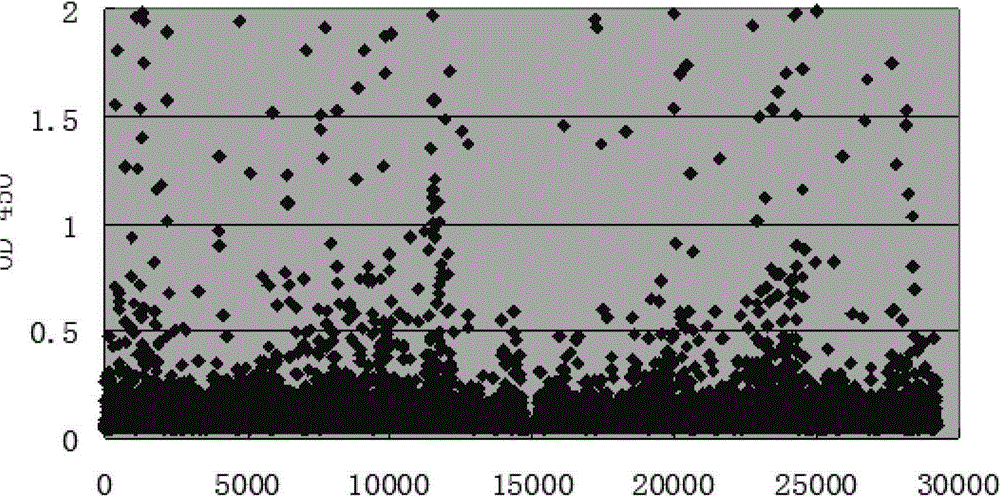
[0109] Example 2: Preparation and identification of monoclonal antibodies
[0110] 1. Preparation of antibodies by in vitro culture method
[0111] The 1.1.71, 1.5.58, 1.6.17, 1.24.2, 2.2.108, 2.39.45, 3.85.3, 3.4.32, 3.12.78, 1.1.71, 1.5.58, 1.6.17, 1.24.2, 2.2.108, 2.39.45, 3.85.3, 3.4.32, 3.12.78, DMEM medium containing 15% fetal bovine serum 3.76.109 cells, gradually reduce the serum to serum-free or serum-free medium containing only a small amount of serum (Invitrogen, 12338-026), put it in 225cm 2 Square bottle, according to inoculation 1×10 5 Cells / ml, inoculate 100ml, inoculate 4 bottles for each cell line.
[0112] 2. Antibody purification
[0113] Take out at 225cm 2 The supernatant of cells cultured in the square flask for about 10 days was centrifuged to remove cell debris, and the antibody was purified by rProteinA affinity chromatography. The cell culture supernatant with pH 7.4 was filtered through a 0.22 μm filter membrane and loaded directly, and then washed with ...
Embodiment 3
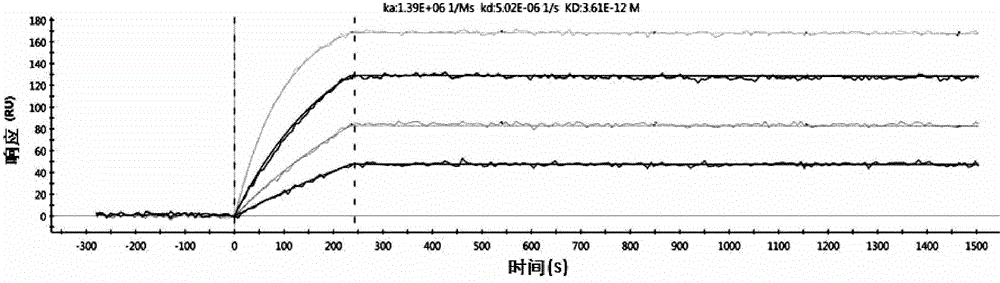
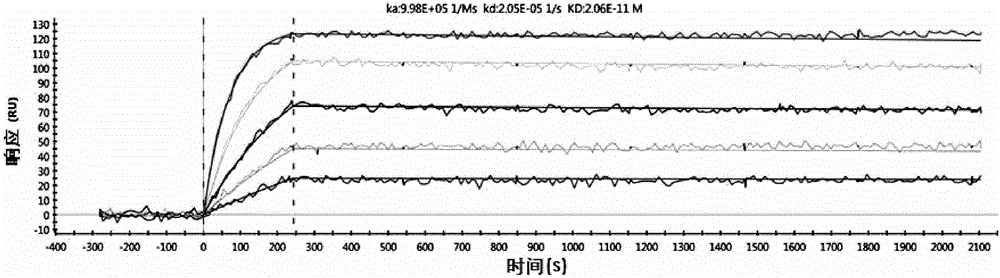
[0114] 3. Example 3: Affinity maturation
[0115] In order to improve the affinity of the antibody, the constructed single-chain antibody scFv is used as a template to perform random mutations, and the mutant single-chain antibody is phage-displayed, and then screened by ELISA to obtain antibodies with higher affinity.
[0116] 3.1 Sequencing the heavy and light chains of Anti-TNFα
[0117] After immunization, fusion and monoclonalization, the anti-TNFα monoclonal antibody cell line was selected as the template for the antibody affinity maturation experiment based on the results of affinity and cell function experiments.
[0118] Sequence analysis of antibody gene heavy and light chains. The total RNA of anti-TNFα monoclonal antibody cells uses Invitrogen's reagent kit (15596-026) for extraction; then use the random primers in Takara's 5'RACE FULL kit (D315), using total RNA as template, reverse transcription into the first strand of eDNA, and follow the kit Operating steps in the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com