NADPH-cytochrome P450 reducing ferment and application thereof
A technology of nicotinamide adenine and cytochrome, which is applied in the fields of biotechnology and plant biology, can solve the problems that the catalytic activity needs to be improved and the kinship is far away.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1, the cloning of nicotinamide adenine dinucleotide (NADPH)-cytochrome P450 reductase
[0062] The four primers synthesized respectively have the nucleotide sequences of SEQ ID NO:3, SEQ ID NO:4, SEQ ID NO:5, and SEQ ID NO:6 in the sequence listing.
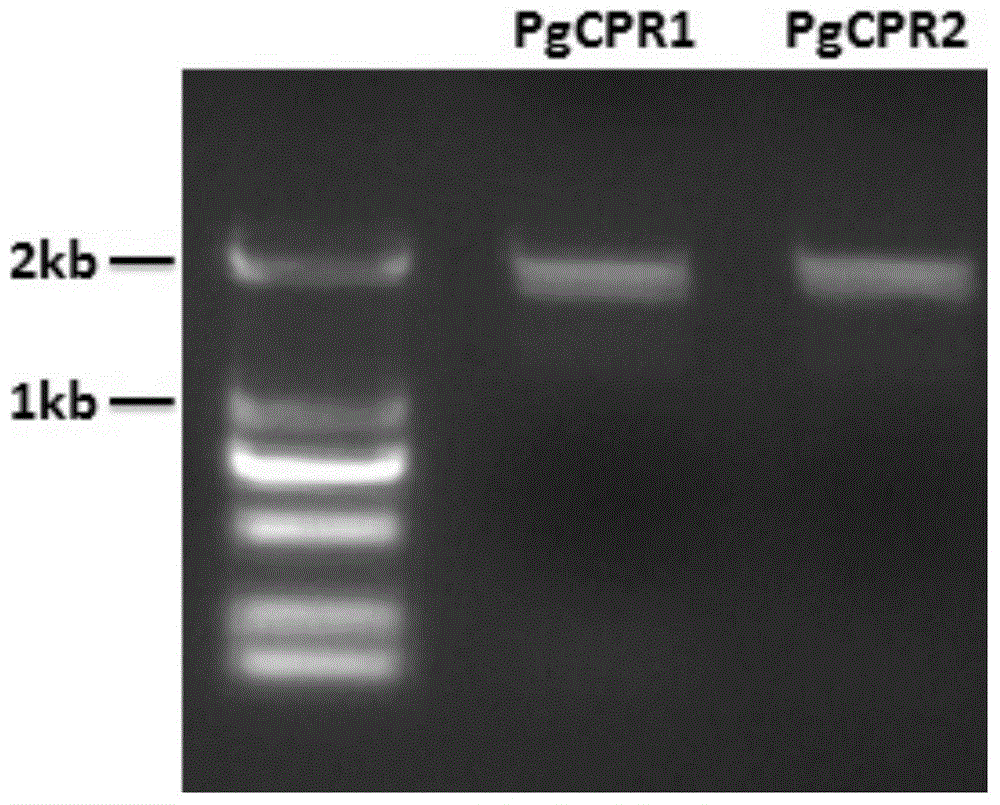
[0063] Using the cDNA obtained by reverse transcription of RNA extracted from ginseng as a template, PCR was performed using the above two pairs of primers SEQ ID NO: 3 / 4 and SEQ ID NO: 5 / 6, respectively. The DNA polymerase was selected from the high-fidelity KOD DNA polymerase of Treasure Bioengineering Co., Ltd. The PCR amplification program was as follows: 94°C for 2min; 94°C for 15s, 58°C for 30s, 68°C for 2min, a total of 35 cycles; 68°C for 10min, then drop to 10°C. The PCR products were detected by agarose gel electrophoresis, and the results were as follows: figure 1 .
[0064]Under UV irradiation, the target DNA band is excised. Then, Axygen Gel Extraction Kit (AXYGEN Company) was used to recover DN...
Embodiment 2
[0067] Example 2, Construction of recombinant vectors expressing PgDDS, CYP716A47, PgCPR1 and PgCPR2 genes
[0068] (1) Synthesizing two primers respectively having the nucleotide sequences of SEQ ID NO:7 and SEQ ID NO:8 in the sequence listing.
[0069] Two restriction sites, BamH I and Xho I, were respectively set at both ends of the synthetic primers SEQ ID NO:7 and SEQ ID NO:8 (amplification of CYP716A47), and PCR was performed using ginseng cDNA as a template. The PCR amplification procedure is the same as in Example 1. The PCR product was separated and recovered by agarose gel electrophoresis, and after being digested by BamH I and Xho I, the T4 DNA ligase of NEB Company was used to connect into the pESC-HIS vector (Agilent Technologies). The obtained recombinant plasmid was named pESC-HIS-CYP.
[0070] (2) Synthesizing six primers respectively having the nucleotide sequences of SEQ ID NO: 11-16 in the sequence listing. Using the genome of Saccharomyces cerevisiae BY...
Embodiment 3
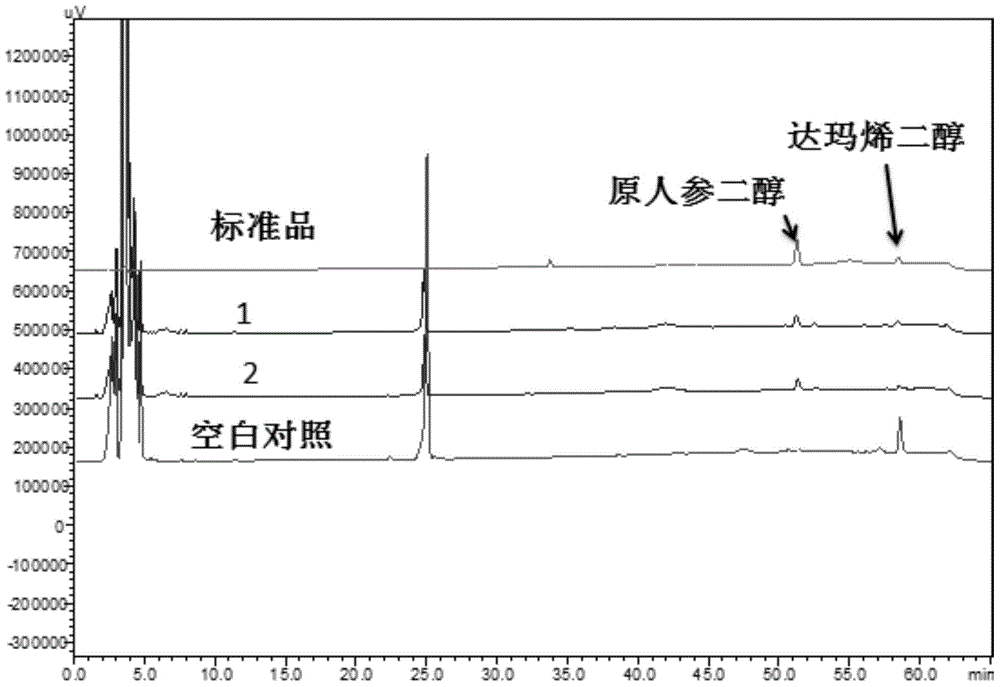
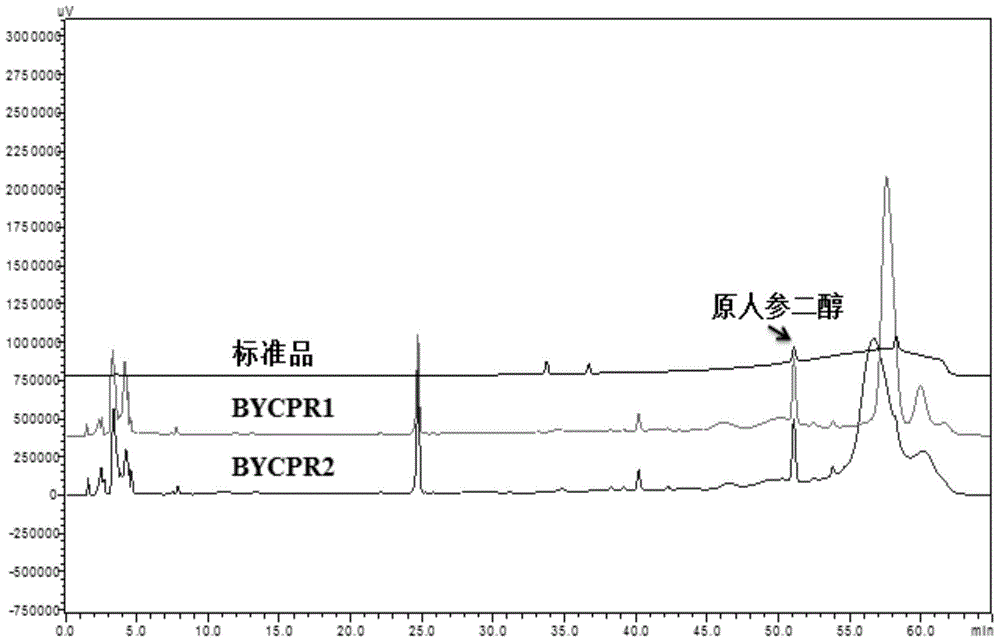
[0080] Example 3. In vitro NADPH-cytochrome P450 reductase and cytochrome P450 (CYP716A47) synergistically catalyze the conversion of dammarenediol to protopanaxadiol
[0081] (1) The recombinant plasmid pHCR1 was introduced into Saccharomyces cerevisiae BY4742 (purchased from Euroscarf) using the Frozen-EZ Yeast Transformation II Transformation Kit of ZYMO Research Company to construct recombinant Saccharomyces cerevisiae BY-CYP-CPR1. Prepare liquid induction medium: 0.67% (w / v) yeast nitrogen source (without amino acids), 2% (w / v) galactose, 0.01% (w / v) leucine, 0.01% (w / v) lysine amino acid, 0.01% (w / v) uracil. Inoculate BY-CYP-CPR1 in 50ml induction medium to induce culture for four days. After collecting the cells by centrifugation, the cells were lysed at low temperature and centrifuged in an ultracentrifuge to prepare microsomes. The recombinant plasmid pESC-HIS-CYP was introduced into BY4742 in the same way to construct recombinant Saccharomyces cerevisiae BY-CYP (bl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com