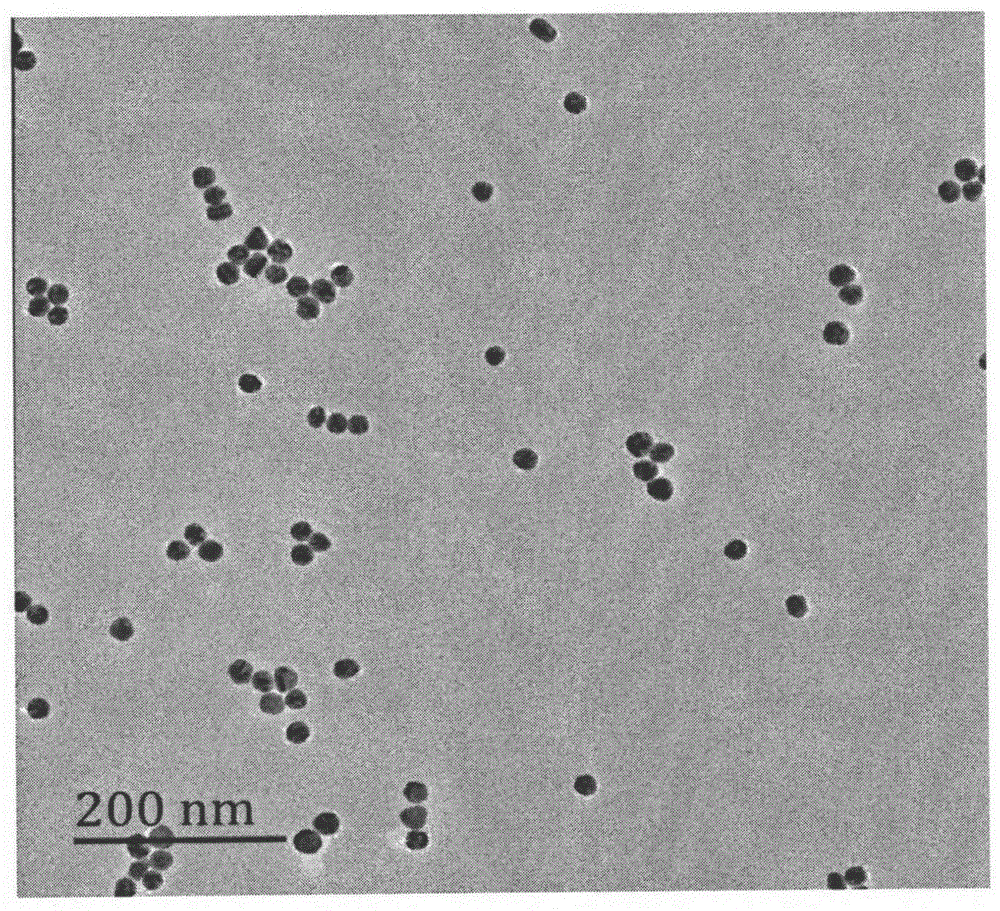
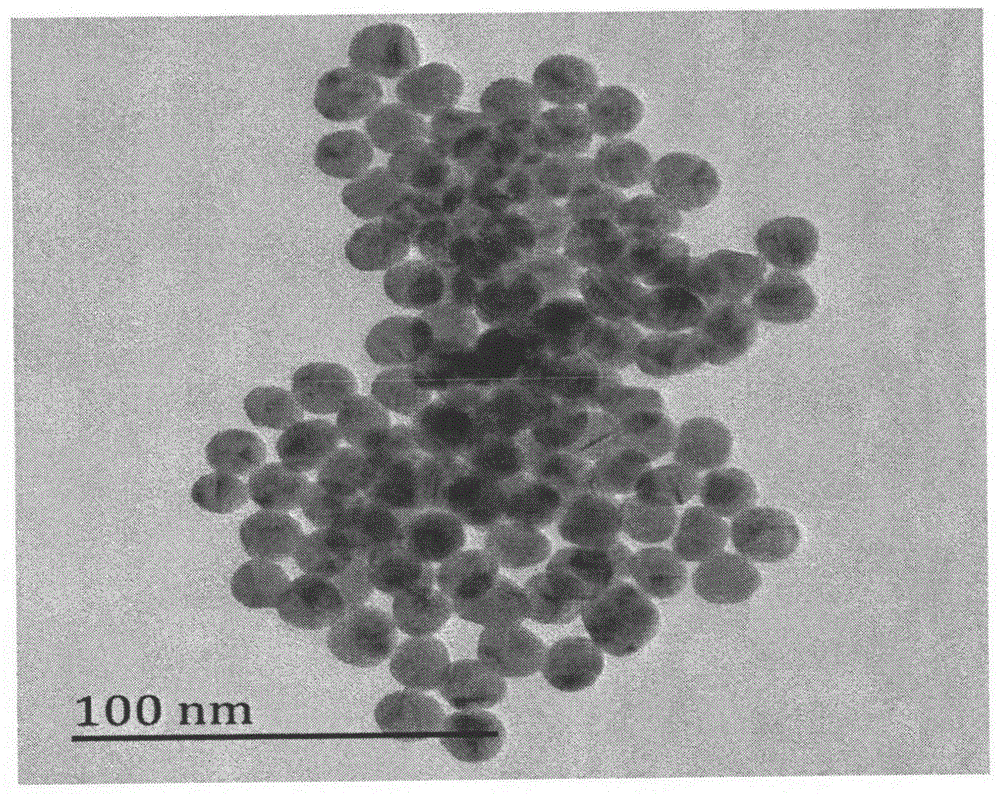
Method for detecting chiral compound based on aptamer modified nanogold
A technology for chiral compounds and nucleic acid aptamers, which is used in the fields of chiral identification, colorimetric analysis and environmental detection, and can solve the problems of long analysis time, unfavorable rapid identification, expensive chiral chromatographic columns, and high separation conditions. , to reduce the detection cost and the complexity of the detection scheme, the modification is convenient and fast, and the effect of non-immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Qualitative and quantitative chiral recognition of S-ibuprofen in the environment with S-ibuprofen aptamer-probe complex
[0029] (1): Preparation of gold nanoparticle solution:
[0030] Add 50mL of ultrapure water into the 100mL round bottom beaker soaked and cleaned by aqua regia, pipette 0.5mL of 1% chloroauric acid aqueous solution prepared in advance into 50mL of ultrapure water with a cleaned 1mL pipette, The concentration of chloroauric acid in the solution was reduced to 0.01% (w / v), and the solution was heated in an oil bath at a constant temperature of 92±4°C. Under the vigorous stirring of the magnet, quickly add 1.6mL of 1% (w / v) trisodium citrate solution prepared in advance, and continue to stir. In the first three minutes of the reaction, the solution basically does not change. After 3 minutes, the solution begins to Turn blue, turn into blue-purple after a few minutes, continue to stir at constant temperature, the solution turns red after 20 ...
Embodiment 2
[0039] Example 2: Qualitative and quantitative chiral recognition of R-ibuprofen in the environment with R-ibuprofen aptamer-probe complex
[0040] (1): Preparation of gold nanoparticle solution: the specific steps are the same as in Example 1.
[0041] (2): prepare the R-ibuprofen aptamer, the sequence of the R-ibuprofen aptamer is:
[0042] 5'-SH GCGAACGACTTCATAAAATGCTATAAGGTTGCCCTCTGTC-3'. After centrifuging the obtained R-ibuprofen aptamer, add Tris-HCl buffer solution to dissolve into a 1 μmol / L solution, and put it in a 4°C refrigerator for later use;
[0043] (3): Using the R-Ibuprofen aptamer as the recognition probe for R-Ibuprofen, add 200 μL of the above-mentioned nano-gold solution and 100 μL of the above-mentioned R-Ibuprofen aptamer solution in a 2 mL centrifuge tube, Add 0, 20, 40, 60, 80, 100, and 120 μL of 100 μmol R-ibuprofen standard solution to 10 μL of 1M sodium chloride solution, and dilute to 500 μL with ultrapure water to obtain a standard detection s...
Embodiment 3
[0049] Example 3: Qualitative and quantitative chiral recognition of S and R-Ibuprofen mixtures with S-Ibuprofen aptamer-probe complex
[0050] (1): Preparation of gold nanoparticle solution: the specific steps are the same as in Example 1.
[0051] (2): Prepare the S-ibuprofen aptamer, the specific steps are the same as in Example 1.
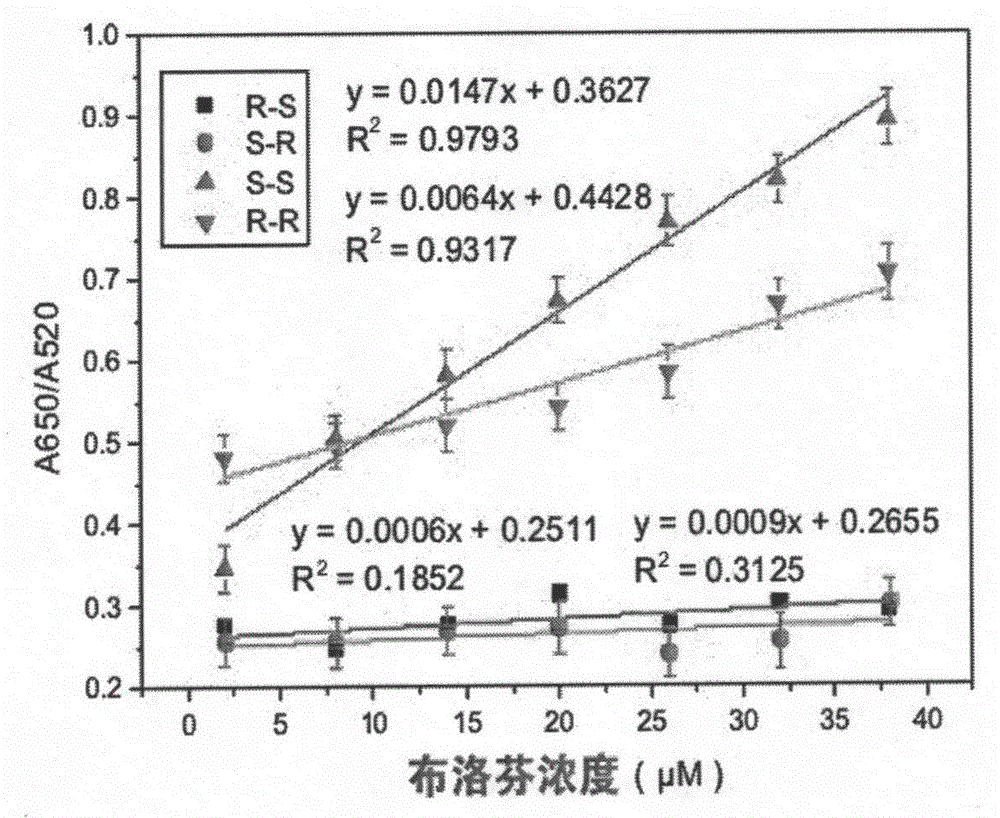
[0052] (3) Using the S-Ibuprofen aptamer as the recognition probe for S-Ibuprofen, add 200 μL of the above-mentioned gold nanoparticle solution and 100 μL of the above-mentioned S-Ibuprofen aptamer solution in a 2 mL centrifuge tube, To 10 μL of 1M sodium chloride solution, add 0, 20, 40, 60, 80, 100, and 120 μL of S-ibuprofen solution with a concentration of 100 μmol and correspondingly add 120, 100, 80, 60, 40, 20, and 0 μL of 100 μmol of R-ibuprofen solution was mixed, and the volume was adjusted to 500 μL with ultrapure water. After reacting for 30 minutes at room temperature, scan with UV-vis in the wavelength range of 190-800nm. Accordi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com