Sodium alginate microsphere vascular embolization agent and preparation method thereof
The technology of a vascular embolizing agent and sodium alginate is applied in the field of sodium alginate microsphere vascular embolizing agent and its preparation, which can solve the problems of tissue or organ embolism, wide product particle size distribution, uncontrollable size, etc., and reduce toxic and side effects. , Improve local efficacy and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This embodiment provides a sodium alginate microsphere vascular embolization agent, which is prepared by the following steps:
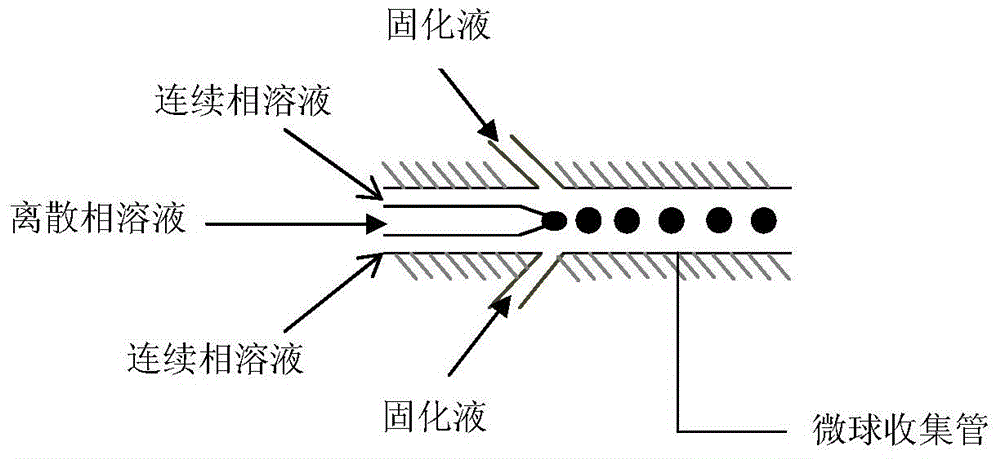
[0037] choose as figure 1 In the microfluidic device shown, the outlet diameter of the discrete phase channel is 150 microns, and the width of the continuous phase channel is 1000 microns; the concentration of the sodium alginate aqueous solution used is 4%, and the solidification solution is 10wt% chloride Calcium aqueous solution;
[0038] The sodium alginate aqueous solution and the solidification solution were pumped into the discrete phase channel at a constant flow rate of 0.3mL / h and 2mL / h, while paraffin oil was pumped into the continuous phase channel at a constant flow rate of 2mL / h; the droplets in the discrete phase Generated near the exit of the channel, while solidifying to form microspheres;
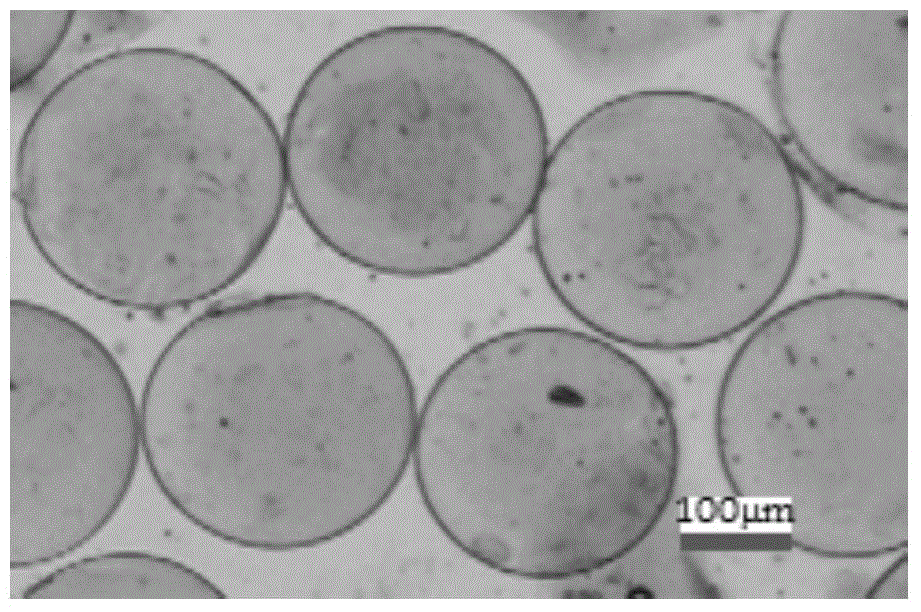
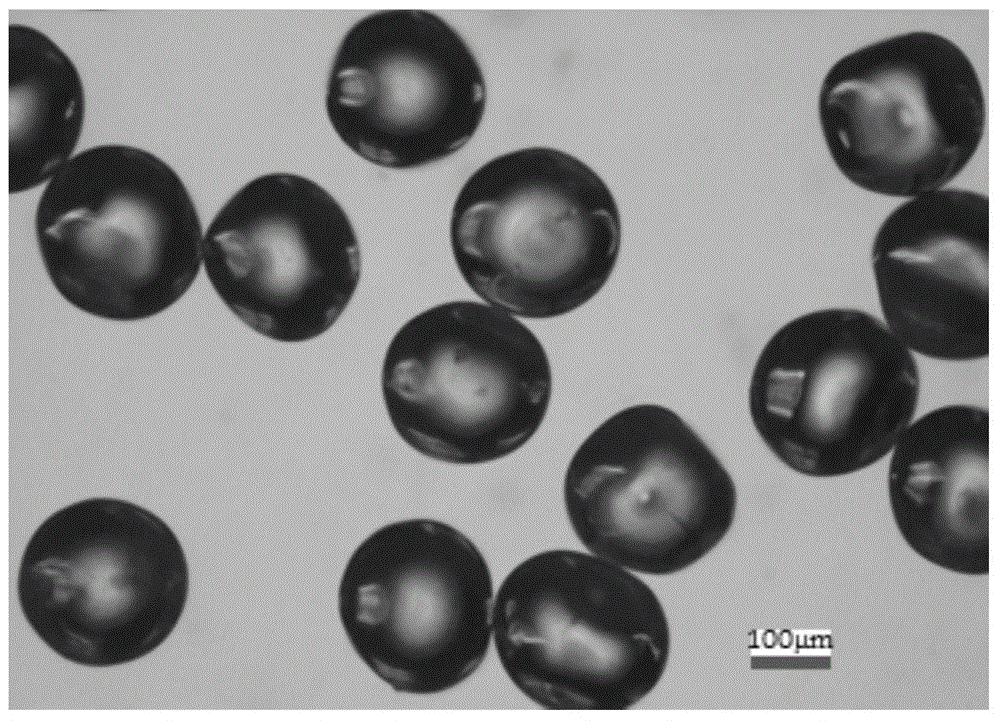
[0039] The microspheres were collected and left to stand at room temperature at 25°C for more than 30 minutes to obtain a white solid; the...
Embodiment 2
[0041] This embodiment provides a sodium alginate microsphere vascular embolization agent, which is prepared by the following steps:
[0042] choose as figure 1 In the microfluidic device shown, the outlet diameter of the discrete phase channel is 160 microns, and the width of the continuous phase channel is 1000 microns; the concentration of the sodium alginate aqueous solution used is 5%, and the solidification solution is 10 wt% chloride Calcium aqueous solution;
[0043] The sodium alginate aqueous solution and the solidification solution were pumped into the discrete phase channel at a constant flow rate of 0.2mL / h and 3mL / h, and soybean oil was pumped into the continuous phase channel at a constant flow rate of 4mL / h; the droplets in the discrete phase Generated near the exit of the channel, while solidifying to form microspheres;
[0044] Collect the microspheres and let them stand at room temperature at 25°C for more than 30 minutes to obtain a white solid; then filt...
Embodiment 3
[0046] This embodiment provides a sodium alginate microsphere vascular embolization agent, which is prepared by the following steps:
[0047] choose as figure 1 In the microfluidic device shown, the outlet diameter of the discrete phase channel is 190 microns, and the width of the continuous phase channel is 1000 microns; the concentration of the sodium alginate aqueous solution used is 1%, and the solidification solution is 10 wt% chloride Calcium aqueous solution;
[0048] The sodium alginate aqueous solution and the solidification solution were pumped into the discrete phase channel at a constant flow rate of 0.05mL / h and 0.4mL / h, while dodecane was pumped into the continuous phase channel at a constant flow rate of 6mL / h; Formed near the outlet of the discrete phase channel, and solidified to form microspheres at the same time;
[0049] Collect the microspheres and let them stand at room temperature at 25°C for more than 30 minutes to obtain a white solid; then filter to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com