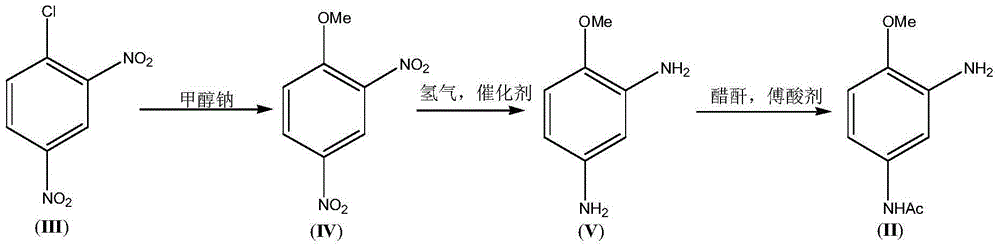
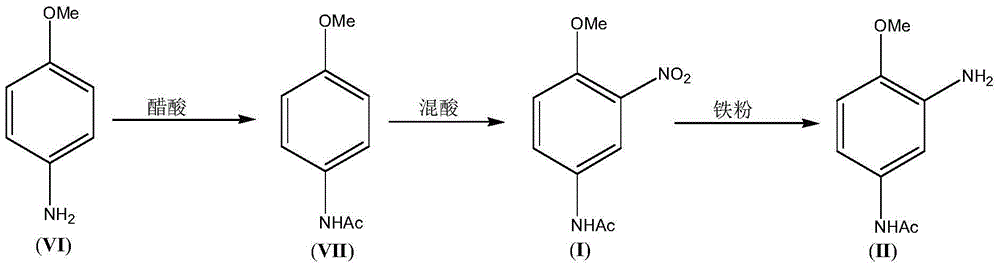
3-Amino-4-methoxy acetanilide (II) preparation method
A technology of methoxyacetanilide and amino, which is applied in the field of preparation of organic compounds, can solve the problems of low catalyst application times, large environmental pollution, and high cost, and achieves convenient storage and transportation, accurate measurement, small investment in equipment and workshops, and avoids The effect of tar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
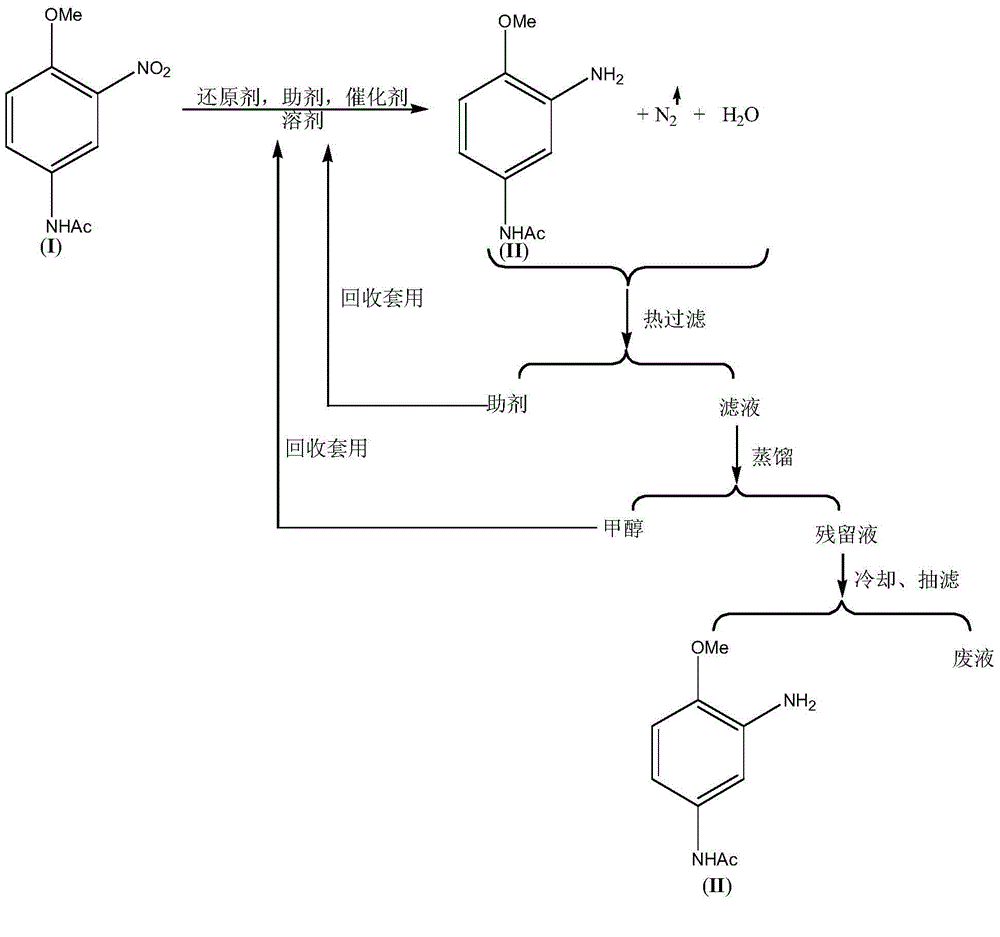
[0029] Embodiment 1: Add 315.3g 3-nitro-4-methoxyaniline (I), 252.2g methyl alcohol, 3.15g FeCl3.6H2O, 6.3g diatomaceous earth successively in four-necked round bottom flask, stir, be heated to 70° C., 154.7 g of hydrazine hydrate with a mass percentage of 80% was added in about 2.0 hours, and the reaction was completed.
[0030] Filtrate hot, recover diatomaceous earth and apply it to the next batch of reactions. Distill and recover 252g methanol, cool and filter with suction to obtain 225.4g (99.3%) 3-amino-4-methoxyaniline (II) and 168g waste water.
[0031] The NMR data of the 3-amino-4-methoxyaniline (II) compound are as follows: 1HNMR (500MHz, CDCl3): δ7.25~7.28(br., 1H), 6.83~6.85(t, 2H) , 6.62 (d, 1H), 3.78 (s, 3H), 3.4-3.6 (br., 2H), 2.03 (s, 3H).
Embodiment 2
[0032] Embodiment 2: Add 210.2g 3-nitro-4-methoxyaniline (I), 420.4g chlorobenzene, 0.21g FeSO4.7H2O, 1.05g silica gel successively in four-necked round bottom flask, stir, be heated to 80 °C, add 93.75 g of hydrazine hydrate with a mass percentage content of 80% in about 3.0 hours, and the reaction is completed.
[0033] Filtrate hot, recover the silica gel, cool, and press filter to obtain 149.4 g (99.0%) of 3-amino-4-methoxyaniline (II).
[0034] The filtrate was allowed to stand for stratification to obtain 413g chlorobenzene and 113g waste water.
Embodiment 3
[0035] Embodiment 3: Add 210.2g 3-nitro-4-methoxyaniline (I), 315g n-hexane, 2.1g Fe2O3, 4.2g gac successively in four-necked round bottom flask, stir, be heated to 40 ℃, in Add 125 g of hydrazine hydrate with a mass percent content of 60% in about 1.0 hour, and the reaction is completed.
[0036] Filtrate hot, recycle activated carbon, cool, and press filter to obtain 151.9 g (99.6%) of 3-amino-4-methoxyaniline (II).
[0037] The filtrate was left to stand and separated to obtain 305g of normal hexane and 143g of waste water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com