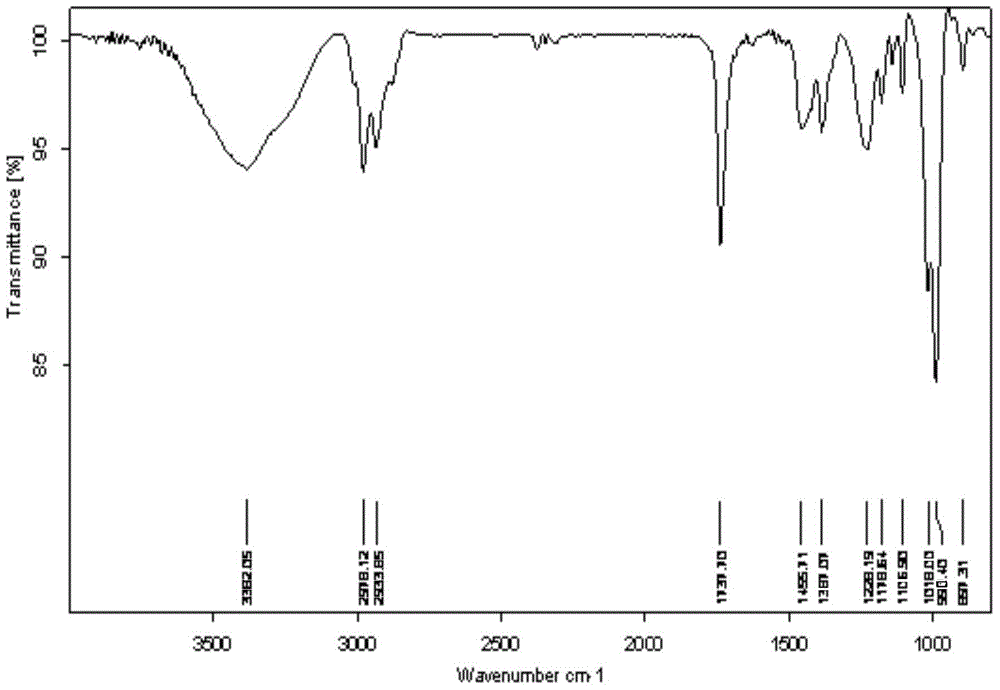
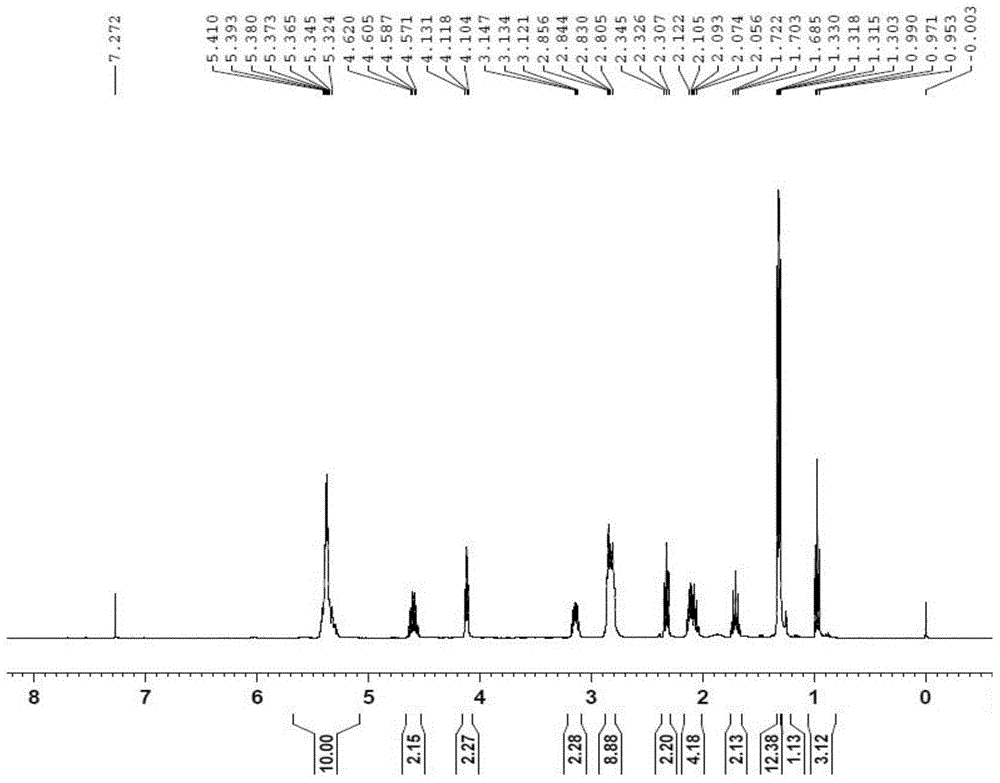
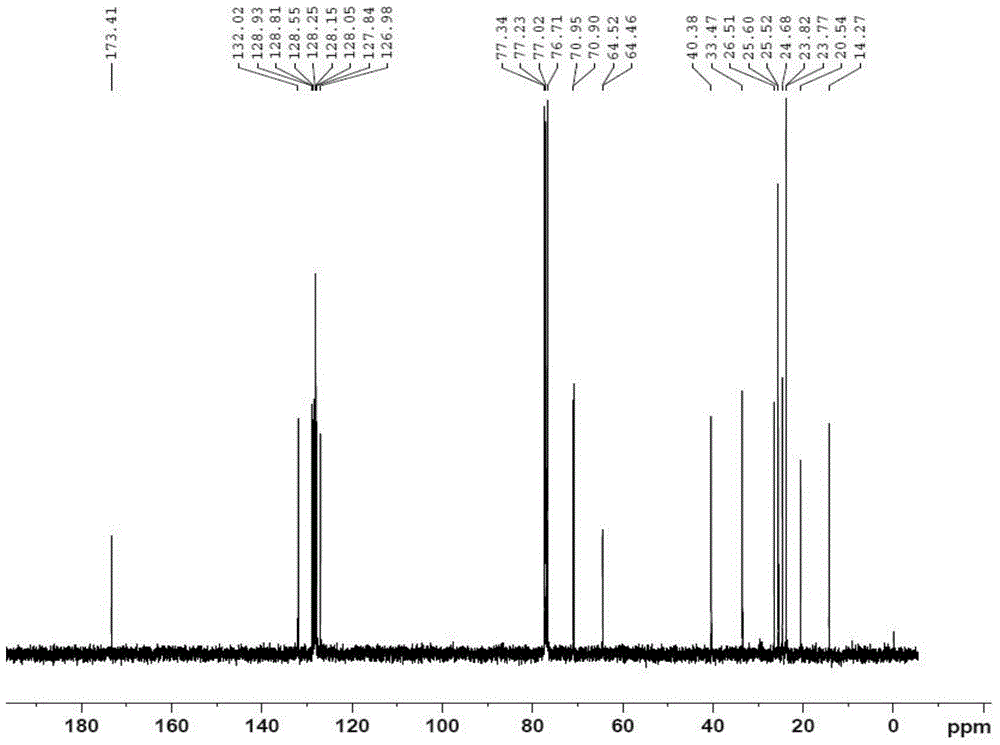
Polyunsaturated fatty acyl phosphatide derivative and preparation method and application thereof
A technology of fatty acyl phospholipids and derivatives, which is applied in the field of series derivatives of compounds, can solve the problems such as great difference in drug efficacy, and achieve the effect of novel structure, convenient operation and prevention of fat accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: prepare DHA-PC (2) compound (2a), the structural formula of compound (2a) is:
[0042]
[0043] Mix 12mmol polyunsaturated fatty acid and 10mmol phosphorylated ethanolamine evenly, and slowly drop into the ester condensing agent, which includes 1.4mL DMF, 3.6mL Et 3 N and 1.2 mL (COCl) 2 30mL dichloromethane mixed solution, stirred at 0°C for 2h. After the reaction finished, the reaction solution was added with saturated sodium carbonate solution (100mL), extracted twice with dichloromethane (50mL), dried over anhydrous magnesium sulfate, concentrated under reduced pressure after filtration, and the thick product was subjected to quick silica gel column chromatography (V 乙酸乙酯 :V 石油醚 =1:2), and purified to obtain a yellow viscous product. Agilent XDB-C18ZORBAX HPLC for further purification, room temperature, mobile phase (V 甲醇 :V 水 =95:5), flow rate 20ml / min, collection retention time t 1 / 2 = the component of 6.1min, promptly obtains light yellow vis...
Embodiment 2
[0046] Embodiment 2: prepare DHA-PC (3) compound (2b), the structural formula of compound (2b) is:
[0047]
[0048] Mix 12mmol polyunsaturated fatty acid and 10mmol phosphorylated propanolamine evenly, and slowly drop into the ester condensing agent, which includes 1.2mL DMF, 3.0mL Et 3 N and 1.0 mL (COCl) 2 30mL dichloromethane mixed solution, stirred at 0°C for 2h. After the reaction finished, the reaction solution was added with saturated sodium carbonate solution (100mL), extracted twice with dichloromethane (50mL), dried over anhydrous magnesium sulfate, concentrated under reduced pressure after filtration, and the thick product was subjected to quick silica gel column chromatography (V 乙酸乙酯 :V 石油醚 =1:2), and purified to obtain a yellow viscous product. Agilent XDB-C18ZORBAX HPLC for further purification, room temperature, mobile phase (V 甲醇 :V 水 =95:5), flow rate 20ml / min, collection retention time t 1 / 2 = 5.9min component, that is, a light yellow viscous produ...
Embodiment 3
[0051] Embodiment 3: prepare DHA-PC (4) compound (2c), the structural formula of compound (2c) is:
[0052]
[0053] Mix 12mmol polyunsaturated fatty acid and 10mmol phosphorylated butanolamine evenly, and slowly drop into the ester condensing agent, which includes 1.6mL DMF, 4.2mL Et 3 N and 1.4 mL (COCl) 2 30mL dichloromethane mixed solution, stirred at 0°C for 2h. After the reaction finished, the reaction solution was added with saturated sodium carbonate solution (100mL), extracted twice with dichloromethane (50mL), dried over anhydrous magnesium sulfate, concentrated under reduced pressure after filtration, and the thick product was subjected to quick silica gel column chromatography (V 乙酸乙酯 :V 石油醚 =1:2), and purified to obtain a yellow viscous product. Agilent XDB-C18ZORBAX HPLC for further purification, room temperature, mobile phase (V 甲醇 :V 水 =95:5), flow rate 20ml / min, collection retention time t 1 / 2 = 6.6min component, that is, a light yellow viscous produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com