Preparation and application of a long-chain non-coding RNA nanosphere
A long-chain non-coding, nano-microsphere technology, which is applied in the direction of non-effective components of oil/fat/wax, gene therapy, genetic material components, etc., can solve the lack of effective means for long-chain non-coding RNA molecules, and long-chain RNA is easy to degrade , Difficulty entering cells and other issues, achieving high transfection efficiency, enhanced stability, and solving preservation problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation scheme of HN-lnc-RNA nanoparticles
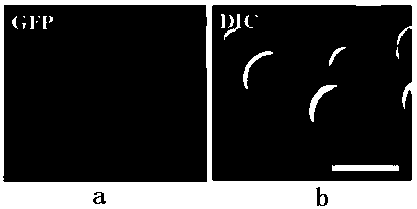
[0024] Dissolve lncRNAHN1 in RNase-free sterilized water to prepare an RNA aqueous solution with a concentration of 200ng~500ng / ml; add vegetable oil to the lncRNA HN1 aqueous solution, the volume ratio of vegetable oil to lncRNA HN1 aqueous solution is 3:2, mix the above vegetable oil with lncRNA Dissolve HN1 in 10 times the volume of distilled water, place the ultrasonic probe at the boundary between water and oil, set the reaction temperature at 4°C, and the ultrasonic intensity at 150 W / cm 2 , and the ultrasonic time was 3 minutes; then, a 20 kDa ultrafiltration membrane was used to separate and remove unbound lncRNA. Its size and particle size distribution were analyzed by DLS and SME methods, see attached figure 1 (b) shown. In order to detect the size and shape of lncRNA-HN1-RNs nanoparticles, lncRNA-HN1-RNs were fixed with 2.5% glutaraldehyde and observed and analyzed under electron microscope, see at...
Embodiment 2
[0025] Example 2: In vitro stability testing of HN-lnc-RNA nanospheres
[0026] The long-chain non-coding RNA HN1 was prepared as lncRNA-HN1-RNs nanospheres, and then placed at room temperature at 25 °C for 7 days. At the same time, lncRNA-HN1 was used as a control sample, and the integrity of the RNA was detected by formaldehyde-denaturing PAGE gel electrophoresis. To evaluate the storage time of RNA nanospheres at room temperature. see attached results figure 2 As shown, the results showed that the untreated lncRNA-HN1 was completely degraded, but the lncRNA-HN1-RNs nanoparticles still showed good integrity after being placed at room temperature for 7 days by electrophoresis detection.
Embodiment 3
[0027] Example 3: Determination of transfection efficiency of HN-lnc-RNA nanospheres
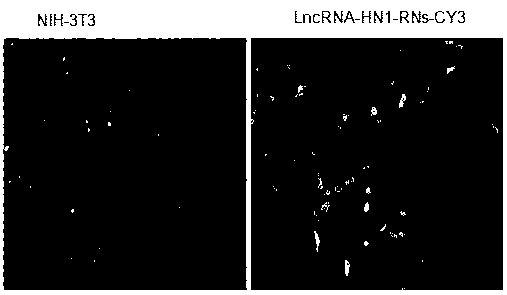
[0028] The 3' end of lncRNA-HN1 was labeled with fluorescent probe Cy3, and prepared as nanospheres according to Example 1, and the prepared lncRNA-HN1-RNs-Cy3 nanospheres were prepared according to the final concentration of 100ng / ml RNA contained. Concentrations were co-incubated with human melanoma cells YU-SIT1 without adding the transfection reagent lipofectamine 2000 and cultured in a 5% CO2 incubator at 37°C for 24 hours, and then the cells were placed under a fluorescence microscope to detect the ratio of green fluorescent cells to evaluate lncRNA - Efficiency of transfecting cells with HN1-RNs nanoparticles. see attached results image 3 As shown, the results showed that after transfection of lncRNA-HN1-RNs-Cy3 nanoparticles, most cells showed fluorescence, indicating that long-chain RNA nanospheres could enter melanoma cells without the aid of transfection reagents.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com