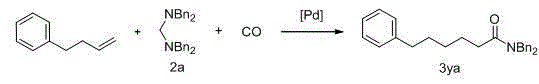Preparation method for fatty acyl amide
A fatty amide and aliphatic-based technology, applied in the field of organic chemical synthesis, can solve the problems of limited substrate applicability, low reaction economy, low atom economy, etc., and achieves wide substrate applicability and clean reaction process. , the effect of reducing the cost of synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 N -Aliphatic substituted amide derivatives 3aa Preparation
[0026] The synthetic route is as follows:
[0027]
[0028] Styrene 1a (91 μL, 0.8 mmol), amine acetal 2a (81.2 mg, 0.2 mmol), Pd(TFA) 2 (3.3 mg, 0.01 mmol), DPPPen (5.3 mg, 0.012 mmol), NH 2 CH 2 CO 2 Me · HCl (5.0 mg, 0.04 mmol), H 2 O (4 μL, 0.22 mmol) is added to 1.0 mL of anisole, carbon monoxide (10 atm), 120 o After C reacted for 21 hours, the reaction was stopped, n-hexadecane was added as the internal standard, the GC yield was 87%, the solvent was evaporated, and the column chromatography was ethyl acetate / petroleum ether (1:10) to obtain the pure amide derivative 3aa . The product was a white solid with a yield of 72%.
[0029] 1 (400 MHz, CDCl 3 ) δ 2.70 (dd, J 1 = 7.4 Hz, J 2 = 10.4 Hz, 2H), 3.03 (dd, J 1 = 7.4 Hz, J 2 = 10.4 Hz, 2H), 4.37 (s, 2H), 4.60 (s, 2H), 7.06 (d, J = 6.9 Hz, 2H), 7.19-7.21 (m, 5H), 7.24-7.35 (m, 8H);
[0030] 13 C NMR (100 MHz, CDCl 3 ) δ 31.6, 35.1...
Embodiment 2
[0032] Example 2 N -Aliphatic substituted amide derivatives 3aa Preparation
[0033] Styrene 1a (91 μL, 0.8 mmol), amine acetal 2a (81.2 mg, 0.2 mmol), PdCl 2 (0.01 mmol), DPPPen (5.3 mg, 0.012 mmol), NH 2 CH 2 CO 2 Me · HCl (5.0 mg, 0.04 mmol), H 2 O (4 μL, 0.22 mmol) is added to 1.0 mL of anisole, carbon monoxide (10 atm), 120 o After C reacted for 21 hours, the reaction was stopped, n-hexadecane was added as an internal standard, and the GC yield was 63%.
Embodiment 3
[0034] Example 3 N -Aliphatic substituted amide derivatives 3aa Preparation
[0035] Styrene 1a (91 μL, 0.8 mmol), amine acetal 2a (81.2 mg, 0.2 mmol), Pd(OAc) 2 (0.01 mmol), DPPPen (5.3 mg, 0.012 mmol), NH 2 CH 2 CO 2 Me · HCl (5.0 mg, 0.04 mmol), H 2 O (4 μL, 0.22 mmol) is added to 1.0 mL of anisole, carbon monoxide (10 atm), 120 o After C reacted for 21 hours, the reaction was stopped, n-hexadecane was added as an internal standard, and the GC yield was 54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com