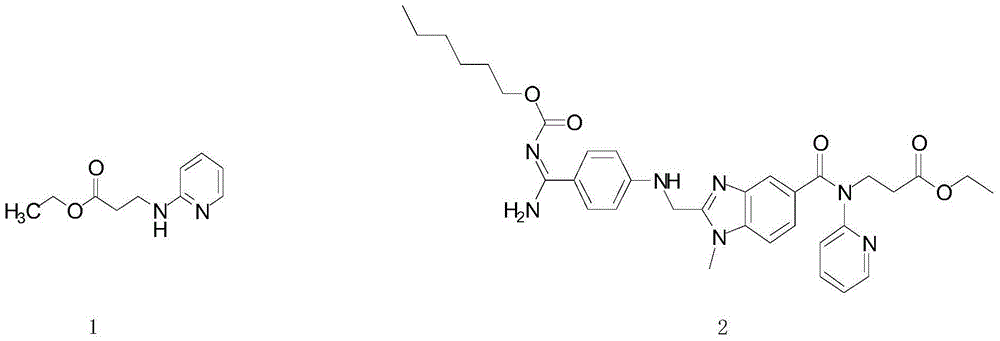
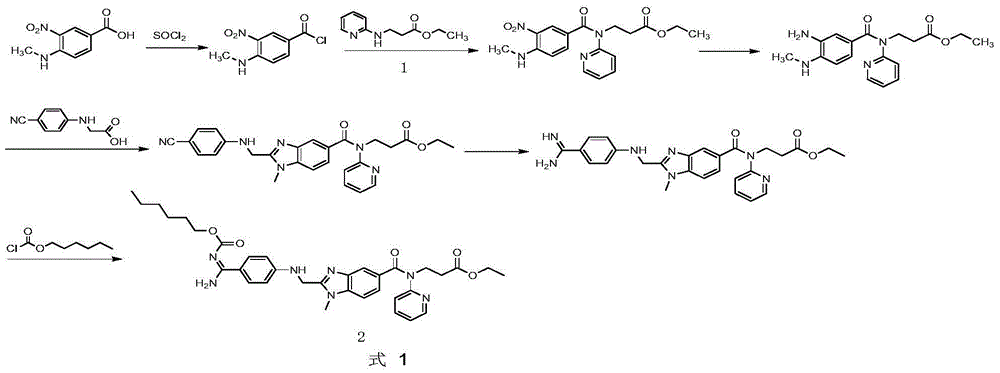
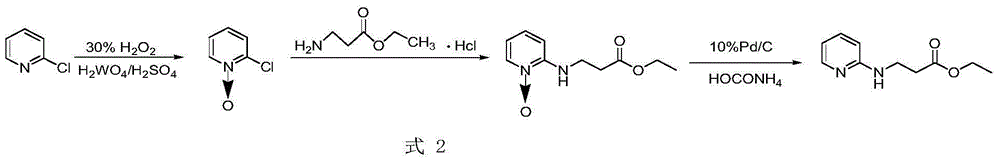
Preparation method for ethyl 3-(pyridin-2-ylamino) propanoate
A technology of ethyl propionate and ethyl acrylate, which is applied in the field of preparation of ethyl 3-propionate, can solve the problems of long reaction time, low yield, complicated reaction process, etc., and achieve short operation time, environmental friendliness, The effect of the simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Put an electromagnetic stirrer bar of appropriate size in a 500mL round bottom flask, weigh 50g of o-aminopyridine into the bottle, and add 50mL of absolute ethanol;
[0035] (2) Stir, and after the solid is dissolved or most of it is dissolved, add 56.5 mL of ethyl acrylate to step (1);
[0036] (3) Stir for a while, slowly add 9 mL of trifluoromethanesulfonic acid dropwise;
[0037] (4) Add nitrogen protection, stir and reflux in an oil bath at 120-160°C for 18 hours;
[0038] (5) After the reaction is completed, the reaction solution is concentrated under reduced pressure after being washed with petroleum ether at a temperature of 35-40°C and a pressure of 0.09-0.1 MPa;
[0039] (7) The concentrated solution was washed with petroleum ether / ethyl acetate (volume ratio 5:1), recrystallized, and suction filtered to obtain white flaky crystals.
[0040] The white flaky crystals are ethyl 3-(pyridin-2-ylamino)propionate with a yield of 80% and a purity of 99% (HPLC)...
Embodiment 2
[0042] (1) Put an electromagnetic stirrer of appropriate size in a 500mL round bottom flask, weigh 100g of o-aminopyridine into the bottle, and add 100mL of absolute ethanol;
[0043] (2) Stir, and until the solid is dissolved or most of it is dissolved, add 113 mL of ethyl acrylate to step (1);
[0044] (3) Stir for a while, slowly drop 15mL trifluoromethanesulfonic acid;
[0045] (4) Add nitrogen protection, stir and reflux in an oil bath at 120-160°C for 20 hours;
[0046] (5) After the reaction is completed, the reaction solution is concentrated under reduced pressure after being washed with petroleum ether at a temperature of 35-40°C and a pressure of 0.09-0.1 MPa;
[0047] (7) The concentrated solution was washed with petroleum ether / ethyl acetate (volume ratio 8:1), recrystallized, and suction filtered to obtain white flaky crystals.
[0048] The white flaky crystals are ethyl 3-(pyridin-2-ylamino)propionate, with a yield of 83% and a purity of 99% (HPLC).
Embodiment 3
[0050] (1) Put an electromagnetic stirrer of appropriate size in a 1000mL round bottom flask, weigh 150g of o-aminopyridine into the bottle, and add 80mL of absolute ethanol;
[0051] (2) Stir, and until the solid is dissolved or mostly dissolved, add 169.5 mL of ethyl acrylate to step (1);
[0052] (3) Stir for a while, slowly add 25mL trifluoromethanesulfonic acid dropwise;
[0053] (4) Add nitrogen protection, stir and reflux in an oil bath at 120-160°C for 16 hours;
[0054] (5) After the reaction is completed, the reaction solution is concentrated under reduced pressure after being washed with petroleum ether at a temperature of 35-40°C and a pressure of 0.09-0.1 MPa;
[0055] (7) The concentrated solution was washed with petroleum ether / ethyl acetate (volume ratio 10:1), recrystallized, and suction filtered to obtain white flaky crystals.
[0056] The white flaky crystals are ethyl 3-(pyridin-2-ylamino)propionate, with a yield of 85% and a purity of 99% (HPLC).
[005...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com