Process for enzymatic synthesis of cefprozil
A technology of enzymatic synthesis of cefprozil, which is applied to the direction of immobilized on/in organic carrier, fermentation, etc., can solve the problems of difficult screening and evaluation of immobilized enzymes, cumbersome production process steps, long reaction time, etc. The effect of shortened time, good product quality and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
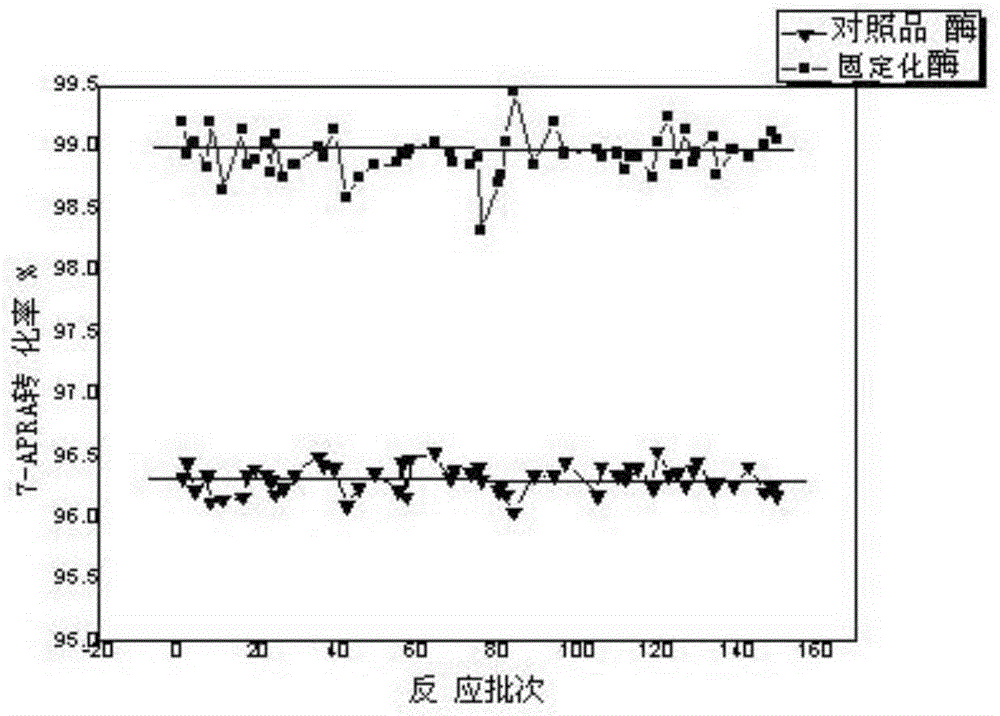
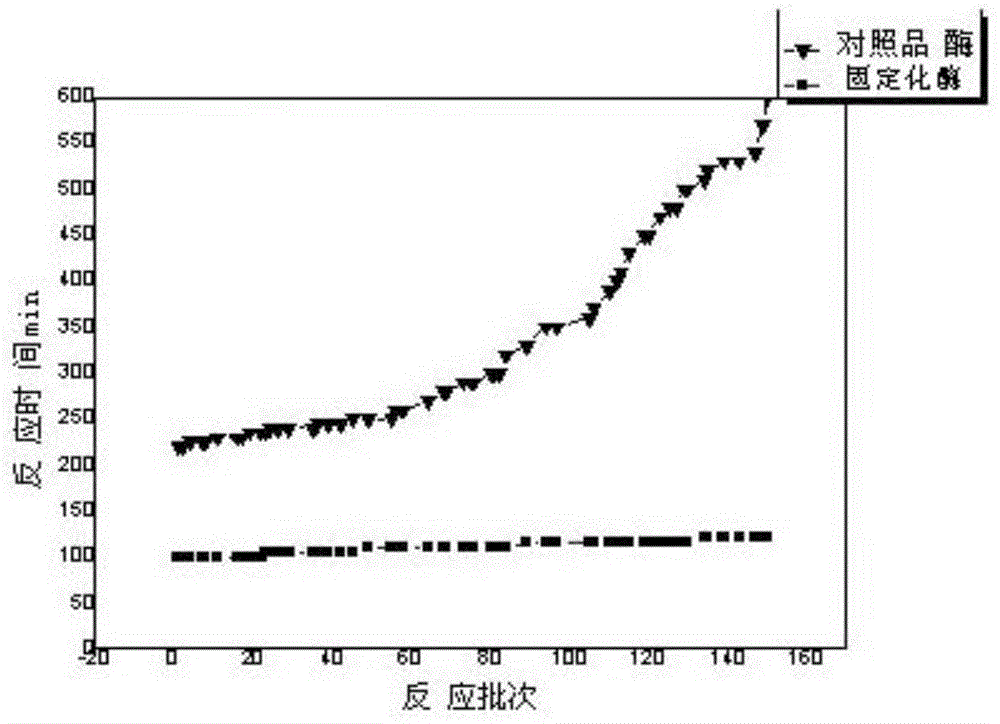
Image
Examples
Embodiment 1
[0034] In the reactor, 3900 u of immobilized cefprozil synthase was added to 130 mL of deionized water, and the stirring was started. 0.04 mol of 7-APRA and 0.046 mol of D-p-hydroxyphenylglycine methyl ester were added. Sodium hydroxide was added to adjust the reaction pH to 6.8. The reaction temperature was maintained at 19-20°C. React for 100min. The reaction solution was separated from the immobilized cefprozil synthetase. The obtained product is adjusted to pH 1.1-1.2 with 30% hydrochloric acid, dissolved, filtered, and then adjusted to pH with 20% ammonia solution to crystallize, and the final pH is 5.0-5.2. After growing the crystal at 0-5°C for 1 hour, washing and drying, the finished product of cefprozil was obtained.
Embodiment 2
[0036] In the reactor, 2600 u of immobilized cefprozil synthase was added to 130 mL of deionized water, and the stirring was started. 0.04 mol of 7-APRA and 0.048 mol of D-p-hydroxyphenylglycine methyl ester hydrochloride were added. Sodium hydroxide was added to adjust the reaction pH to 7.0. The reaction temperature was maintained at 14-15°C. React for 120min. The reaction solution was separated from the immobilized cefprozil synthetase. The obtained product is adjusted to pH 1.1-1.2 with 30% hydrochloric acid, dissolved, filtered, and then adjusted to pH with 20% ammonia solution to crystallize, and the final pH is 5.0-5.2. After growing the crystal at 0-5°C for 1 hour, washing and drying, the finished product of cefprozil was obtained.
Embodiment 3
[0038] In the reactor, 3000 u of immobilized cefprozil synthase was added to 130 mL of deionized water, and the stirring was started. 0.04 mol of 7-APRA and 0.044 mol of D-p-hydroxyphenylglycine methyl ester were added. Sodium hydroxide was added to adjust the reaction pH to 7.1. The reaction temperature was maintained at 10-11°C. React for 120min. The reaction solution was separated from the immobilized cefprozil synthetase. The obtained product is adjusted to pH 1.1-1.2 with 30% hydrochloric acid, dissolved, filtered, and then adjusted to pH with 20% ammonia solution to crystallize, and the final pH is 5.0-5.2. After growing the crystal at 0-5°C for 1 hour, washing and drying, the finished product of cefprozil was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com