Application of galloyl-glucoside derivative and pharmaceutical composition for treating hyperuricemia
A technology of galloyl glucoside and hyperuricemia, applied in the field of application of galloyl glucoside derivatives and pharmaceutical compositions for the treatment of hyperuricemia, can solve the problem of no further animal experiment data, inhibitory effect not obvious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: Extraction and characterization of compound (1)-(3) of the present invention
[0058] Take 10kg of the medicinal material of myrobalan, pulverize it, and extract it twice with water in a slightly boiling state, each time for 40 minutes. Concentrate the extract in vacuo to 3L, extract the concentrate with n-butanol, concentrate and dry the extract under reduced pressure, use Diaion HP-20 macroporous adsorption resin for n-butanol extraction for column chromatography separation, water, 30% ethanol, 60% ethanol and 95% ethanol were used as eluents for elution. The eluent eluted with water and 30% ethanol is separated by column chromatography on reversed-phase C18 silica gel, and eluted with 5%-35% ethanol as the eluent to obtain compounds 1, 2 and 3 of the present invention respectively. , with HPLC-ESI-MS and NMR detection characterization data as follows:
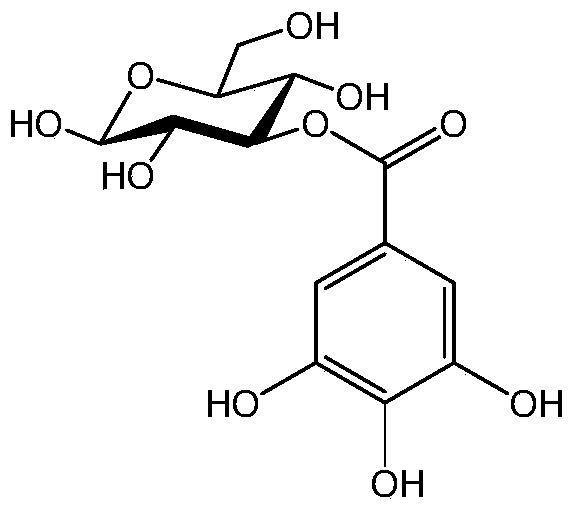
[0059] Compound (1): 3-O-galloyl-β-D-glucopyranose C 13 h 16 o 10 M-H - :331.21
[0060] 1 H-...
Embodiment 2
[0068] Embodiment 2: Extraction and characterization of compound (4)-(6) of the present invention
[0069] Take 5 kg of dried leaves of Potassium officinalis, crush them, and use 60% ethanol to ultrasonically extract twice at 60°C for 40 minutes each time. Concentrate the extract to 2L in vacuo, extract the concentrate with ether, ethyl acetate and n-butanol respectively, concentrate and dry the extract under reduced pressure, and separate the extracted part of ethyl acetate by column chromatography with Diaion HP-20 macroporous adsorption resin , and eluted with water, 30% ethanol, 70% ethanol, and 95% ethanol as eluents. Take 30% and 70% ethanol eluted parts and use reverse phase C18 silica gel for column chromatography separation, use 20-70% ethanol as the eluent for elution to obtain the compounds (4) and (5) of the present invention respectively And (6), detect characterization data with HPLC-ESI-MS and NMR as follows:
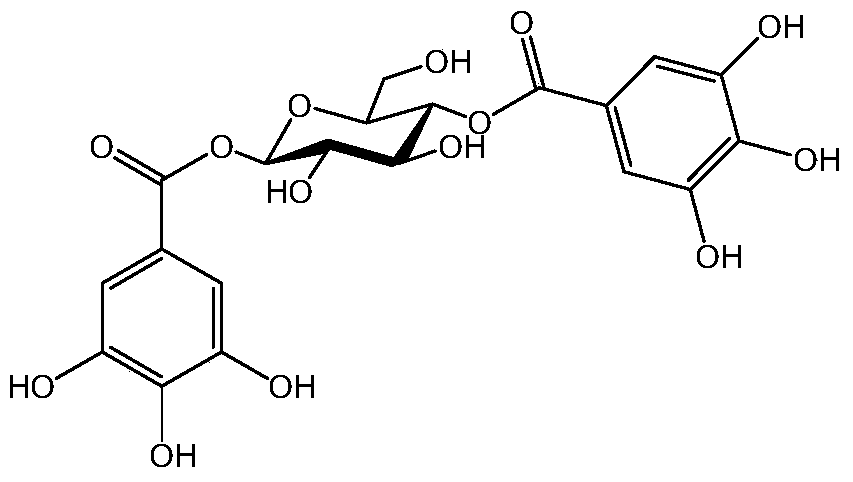
[0070] Compound (4): 1,2,6-tri-O-galloyl-β-D-gluc...
Embodiment 3
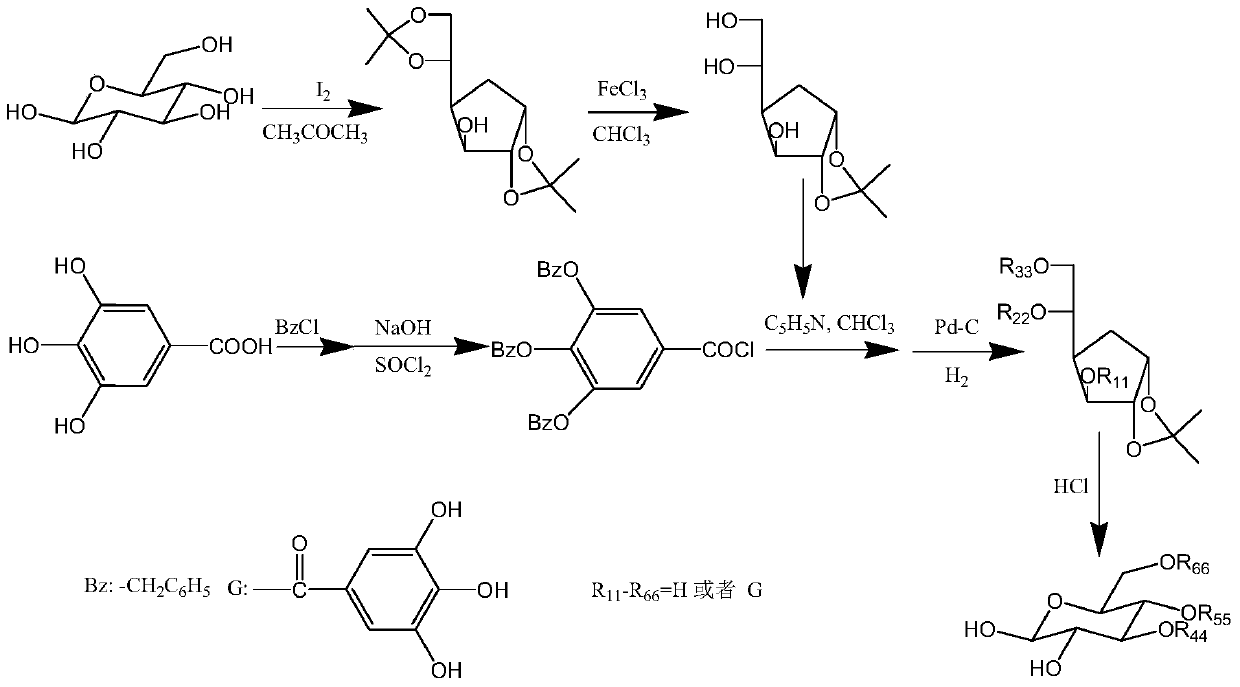
[0079] Embodiment 3: Synthesis and characterization of compound (7)-(9) of the present invention
[0080] Compound (7)-(9) of the present invention adopts such as figure 1 Synthetic by the synthetic route shown.
[0081] The specific operation is as follows:
[0082] 1: Synthesis of 1,2-O-isopropylidene-D-glucofuranose
[0083] Using iodine (11g) as a catalyst, add 40.0g of anhydrous D-glucose to 2000ml of acetone, stir, raise the temperature to 56±1°C, continue the reaction for 2 hours, cool, and add 2% (w / w) sulfur to the reaction solution Sodium sulfite solution 2000ml, the reaction solution changed from yellow to colorless, extracted 3 times with 1000ml chloroform respectively, combined the extracts, then washed the extracts with water, dried over anhydrous sodium sulfate, evaporated the chloroform under reduced pressure to obtain the crude product, and dissolved in benzene: Recrystallization from petroleum ether (1:1, V / V) gave 41 g of colorless needle-like crystals of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com