Preparation method of 2,2'-dihydroxy-4-methoxybenzophenone
A technology of methoxybenzophenone and trihydroxybenzophenone, which is applied in the field of chemical synthesis, can solve the problems of low preparation yield, undisclosed preparation method for product purification, and high cost, so as to improve the preparation efficiency and solve the problem of product Effects of appearance problems, yield and quality improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
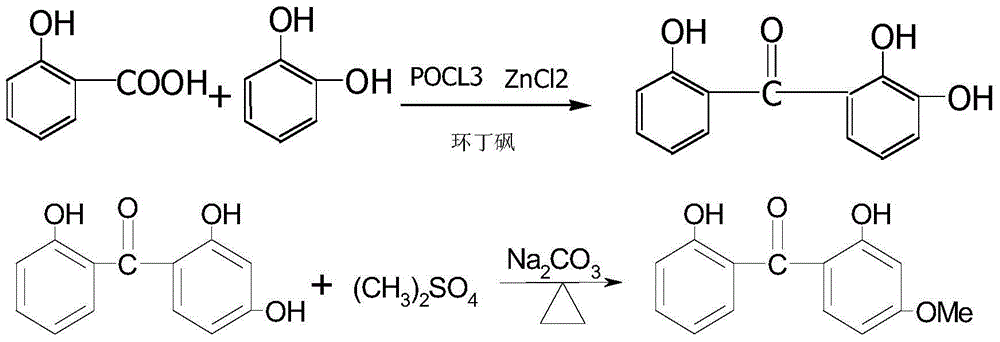
[0027] Weigh 50g of sulfolane, 20mL of phosphorus oxychloride, 30g (0.2mol) of anhydrous zinc chloride in a 250mL four-neck glass bottle, stir and heat to 50°C for 1 hour, then add 15.2g (0.11mol) of salicylic acid, Resorcinol 11.0g (0.1mol), heated up to 75°C and reacted for 2 hours. After the reaction was completed, 20mL of water was added dropwise under cooling to terminate the reaction. The reaction solution was poured into 250mL of ice water and stirred for 5 hours, cooled to crystallize, filtered and dried, and reassembled. 21.3g, the content of 2,2',4-trihydroxybenzophenone detected by HPLC is 96.58%, and the yield is 92.60%;
[0028] Take 21.3g of the prepared 2,2',4-trihydroxybenzophenone in a 250mL reaction flask, add 60mL of toluene, 12.7g (0.12mol) of sodium carbonate, stir and raise the temperature to 45°C, add dropwise dimethyl sulfate 16.4g (0.13mol), keep it warm for 2 hours after dripping, add 50mL of distilled water to wash, stir for 10 minutes and then separ...
Embodiment 2
[0031] Weigh 250g of sulfolane, 100mL of phosphorus oxychloride, 150g (0.2mol) of anhydrous zinc chloride, add to a stirred 1000mL reaction flask, heat to 55°C for 1 hour, then add 72.5g (0.53mol) of salicylic acid , resorcinol 55.0g (0.5mol), heated to 72 ° C for 2 hours, after the reaction was completed, 50 mL of water was added dropwise under cooling to terminate the reaction, the reaction solution was poured into 1000 mL of ice water and stirred for 10 hours, cooled and crystallized, filtered at room temperature The product was collected and weighed 108.3g after drying. The 2,2',4-trihydroxybenzophenone content was 95.20% as detected by GC, and the yield was 94.17%;
[0032] Take 108.3g of the prepared 2,2',4-trihydroxybenzophenone in a 1000mL reaction flask, add 300mL of toluene, 63.5g (0.60mol) of sodium carbonate, stir and raise the temperature to 45°C, add dimethyl sulfate dropwise 82.0 g (0.65 mol), keep it warm for 2 hours after dripping, add 250 mL of distilled wate...
Embodiment 3
[0035] Weigh 500kg sulfolane, 200L phosphorus oxychloride, 300kg (2000mol) anhydrous zinc chloride, add in 1000L enamel reaction tank, heat up to 50 ° C for 1 hour, then add 152kg (1100mol) of salicylic acid, resorcinol 110kg (1000mol), heated at 70-75°C to react for 2 hours. After the reaction, 100L of water was added dropwise to terminate the reaction. The reaction liquid was transferred to a 2000L enamel reaction tank that had been cooled and filled with 1000L of ice water, stirred for 8 hours, cooled and crystallized, and centrifuged. After drying, it weighs 208kg. The content of 2,2',4-trihydroxybenzophenone detected by HPLC is 97.15%, and the yield is 90.43%;
[0036] Take 208kg (904mol) of the prepared 2,2',4-trihydroxybenzophenone into a 2000L enamel reaction tank, add about 600kg of toluene, 127kg (1200mol) of sodium carbonate, stir and heat up to 45°C, add disulfuric acid di Methyl ester 164kg (1300mol), keep warm for 2 hours after dripping, add 30OL water to wash, s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com