Recombinant oncolytic adenovirus and applications thereof
A technology of oncolytic adenovirus and virus, which is applied in the field of biotechnology and gene therapy, can solve the problems affecting the treatment effect, achieve the effect of improving safety, improving anti-tumor ability, and improving tumor killing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of recombinant oncolytic adenovirus shuttle vector plasmid
[0026] 1. Cloning of E1a gene
[0027] According to the human adenovirus type 5 E1a gene sequence (NC_001406) disclosed in GenBank, the following primers were designed to amplify the E1a gene:
[0028] E1a upstream primer: 5'-GCCTGCAGACCACCATGGGACATATTATCTGCCAC-3'
[0029] E1a downstream primer: 5'-GCGGATCCTTATGGCCTGGGGCGTTTACAGC-3'
[0030] Optimize the reaction conditions of each step and the optimal concentration of the reagents involved in the reaction, and perform DNA amplification on the PCR machine. The total volume of the reaction is 50 μL: 5 μL of 10× PCR buffer, 20 μmol / L. 1 μL of each downstream primer, 5 μL of template DNA, 5 μL of dNTPs (2.5 mmol / L each), 25 mmol / L MgCl 2 4μL, 1U / μL Ex-Taq DNA Polymerase 1μL, ddH 2 O 27 μL. Screen and determine the PCR working program: 94°C for 4min; then 94°C for 30s, 57°C for 45s, 72°C for 1min, 10 cycles; finally, 72°C for 10min, and...
Embodiment 2
[0042] Example 2 Preparation of recombinant oncolytic adenovirus
[0043] 1. Co-transfection
[0044] Plasmid pAd-ATV was digested and linearized with Nhe I. The method is as follows: mix an appropriate amount of plasmid DNA with an appropriate amount of water, and add 4U of restriction enzyme Nhe I and 10 μl of the corresponding 10× restriction endonuclease reaction buffer at the same time. , the total volume was 100 μl, the tube wall was flicked to mix and centrifuged, and it was placed in a 37°C water bath overnight.
[0045] Add 100 μl of saturated phenol to the linearized plasmid, shake moderately, and centrifuge at 4°C and 12,000 rpm for 10 min; take the supernatant, add 100 μl of saturated phenol / chloroform / isoamyl alcohol (25:24:1), shake moderately, and place in Centrifuge at 4°C, 12000rpm for 10min; take the supernatant, add 100μl chloroform / isoamyl alcohol (24:1), shake moderately, centrifuge at 4°C, 12000rpm for 10min; take the supernatant, add 200μl absolute etha...
Embodiment 3
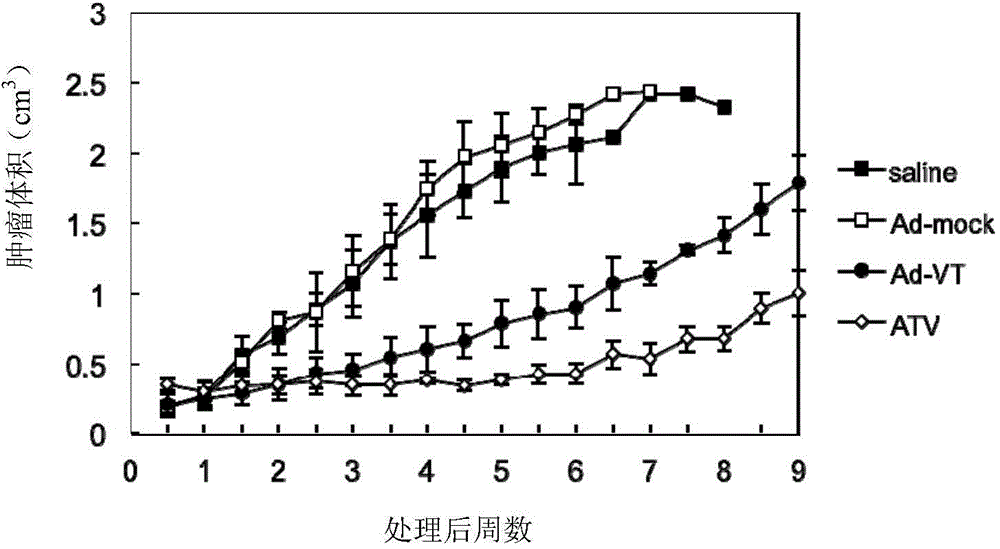
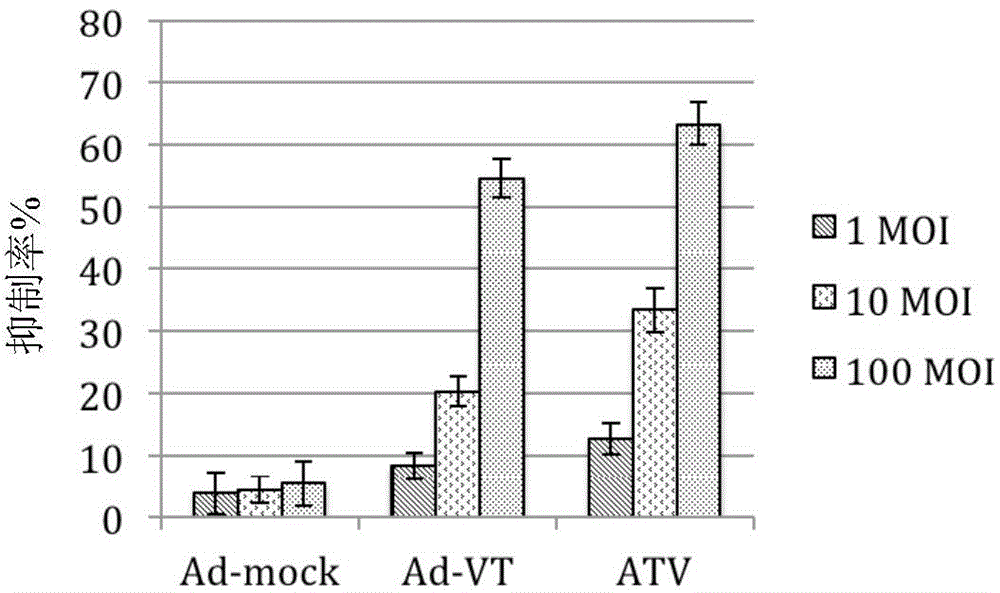
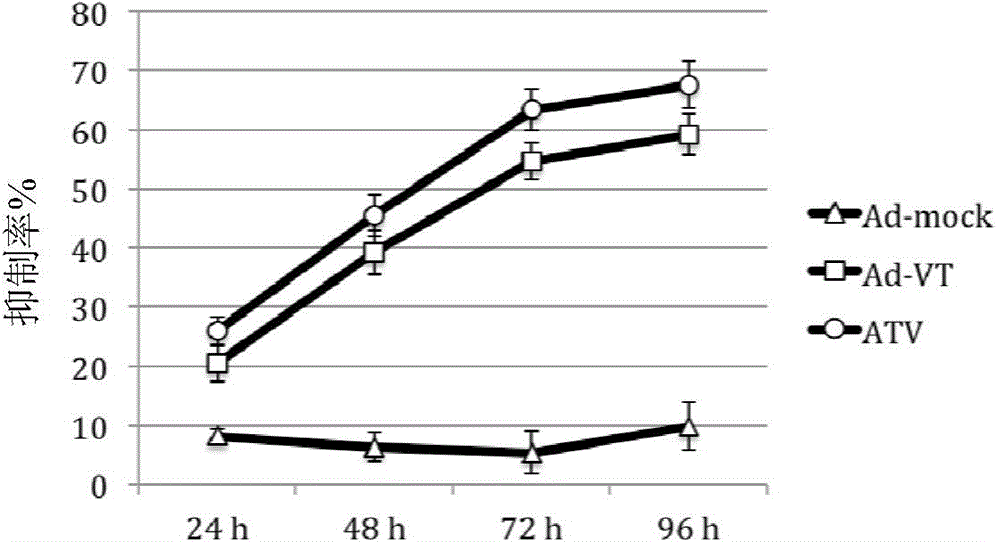
[0057] Example 3 Killing effect of recombinant oncolytic adenovirus on tumor cells (MTT assay)
[0058] Digestion of human lung cancer cells A549 in logarithmic growth phase. Count and adjust the cell concentration to 5 x 10 with complete cell culture medium 4 cells / ml, inoculated in 96-well cell culture plates (ie 5×10 cells) at 100μl / well 3 cells / well), after the cells adhered (about 24h), the culture medium was discarded and washed twice with Hank's solution. Adjust the ATV titer to 1 × 10 with serum-free and antibiotic-free RPMI-1640 medium 7 PFU / ml, 1×10 6 PFU / ml and 1×10 5 PFU / ml. Take 50 μl of the virus dilution prepared in Example 2 (i.e. 100 moi, 10 moi and 1 moi), add them into the corresponding wells of the tumor cells cultured in a 96-well cell culture plate washed with Hank’s solution, and at 37°C, 5% CO. 2 The cells were placed in a cell incubator for 4 hours, and complete RPMI-1640 culture medium was added to 200 μl / well. Untreated cells were used as cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com