Application of ferulic acid derivative as neuroprotective drug
A technology of ferulic acid and its derivatives, applied in the field of medicine, can solve the problems of difficult to penetrate the blood-brain barrier, limit the clinical application of neuroprotective drugs, and low bioavailability, so as to reduce cell free radical damage and achieve significant neuroprotective effects , reduce the effect of cell inflammation damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
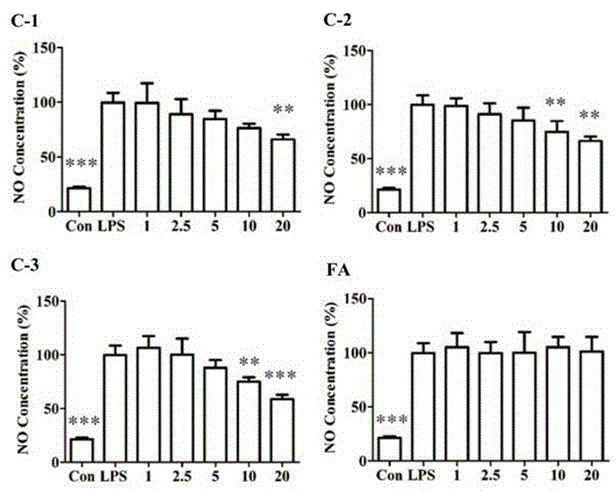
[0024] Example 1: Effect of ferulic acid derivatives on NO.
[0025] Count the RAW264.7 macrophages in the growth phase, and adjust the cell density to 5×10 5 cells / ml, seeded in 96-well plate, 100 μl / well, placed at 37 ℃, 5% CO 2 cultured in an incubator. 20 h after the cells adhered to the wall, the medium in the well was discarded, and 80 μl / well basal medium was added, and 10 μl / well of different concentrations of the test compound was added for treatment, and LPS was added after 1 h, with a final concentration of 1 μg / ml, and the cell supernatant was collected after 20 h. Colorimetric method with NaNO 2 Color development, NO content was detected at a wavelength of 550 nm.
[0026] The results showed that after LPS stimulated RAW264.7 macrophages for 20 h, the NO content increased significantly. Ferulic acid derivatives can inhibit NO production in a dose-dependent manner, but ferulic acid has no inhibitory effect on the increase of NO secretion induced by LPS. se...
Embodiment 2
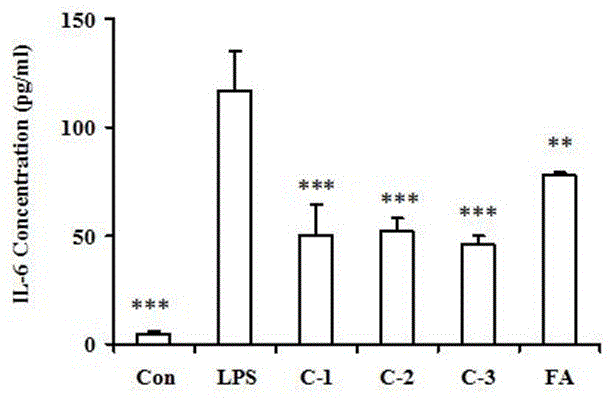
[0027] Example 2: Effects of ferulic acid derivatives on the production of the pro-inflammatory cytokine IL-6.
[0028] Take the RAW264.7 macrophages in the logarithmic growth phase, and adjust the cell density to 5×10 5 cells / ml, seeded in 96-well plate, 100 μl / well, placed at 37 ℃, 5% CO 2 cultured in an incubator. After 20 h of cell attachment, the medium in the well was discarded, and 80 μl / well of basal medium was added, and 10 μl / well of different test compounds (final concentration 10 μM) were added for treatment, and LPS was added after 1 h. The final concentration was 1 μg / ml, and the cell supernatant was collected after 20 h. The concentration of IL-6 was determined by double antibody sandwich ABC-ELISA method.
[0029] The results showed that, compared with the normal group, the content of IL-6 in the culture supernatant of RAW264.7 macrophages only treated with LPS was significantly increased. However, after 1 h of drug pretreatment, 10 μM of ferulic acid der...
Embodiment 3
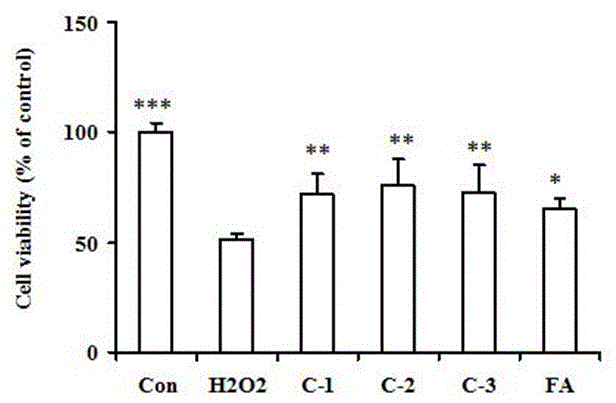
[0030] Example 3: Ferulic acid derivatives on H 2 o 2 Protection against induced neuronal injury.
[0031] Take PC12 cells in the logarithmic growth phase and adjust the cell density to 1 × 10 6cells / ml, seeded in 96-well plate, 100 μL / well, set at 37 ℃, 5% CO 2 Incubate for 24 h in the incubator. The culture medium was replaced, and different test compounds (final concentration 10 μM) were added to the administration group for pretreatment for 1 h, except for the normal group, H 2 o 2 (final concentration 200 μM) caused free radical damage to cells, and cultured for another 24 h. Then, the cell viability of each group was detected according to the MTT method, that is, 15 μL of 5 mg / mL MTT was added to each well, and the culture was continued for 4 h. The supernatant was discarded, and 200 μL of DMSO was added to each well, and the cells were shaken repeatedly for 5 min until blue-purple crystals formed. Dissolve completely, and measure the OD value of each well with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com