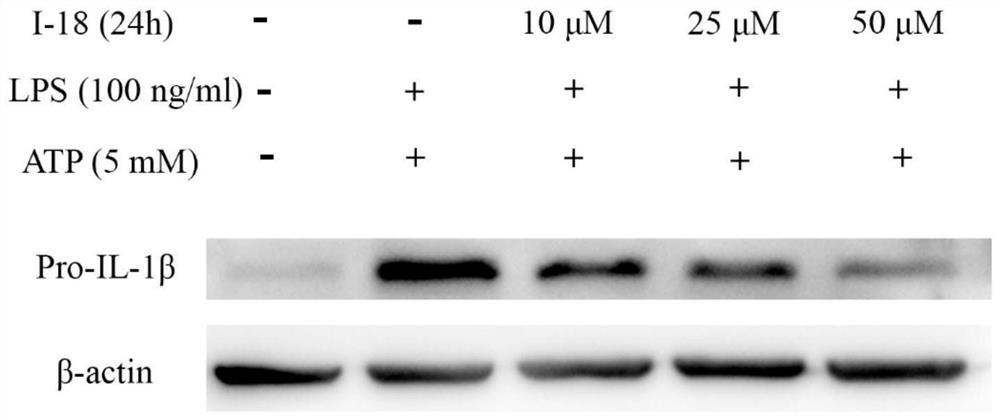
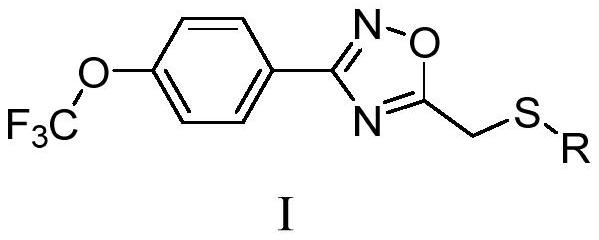
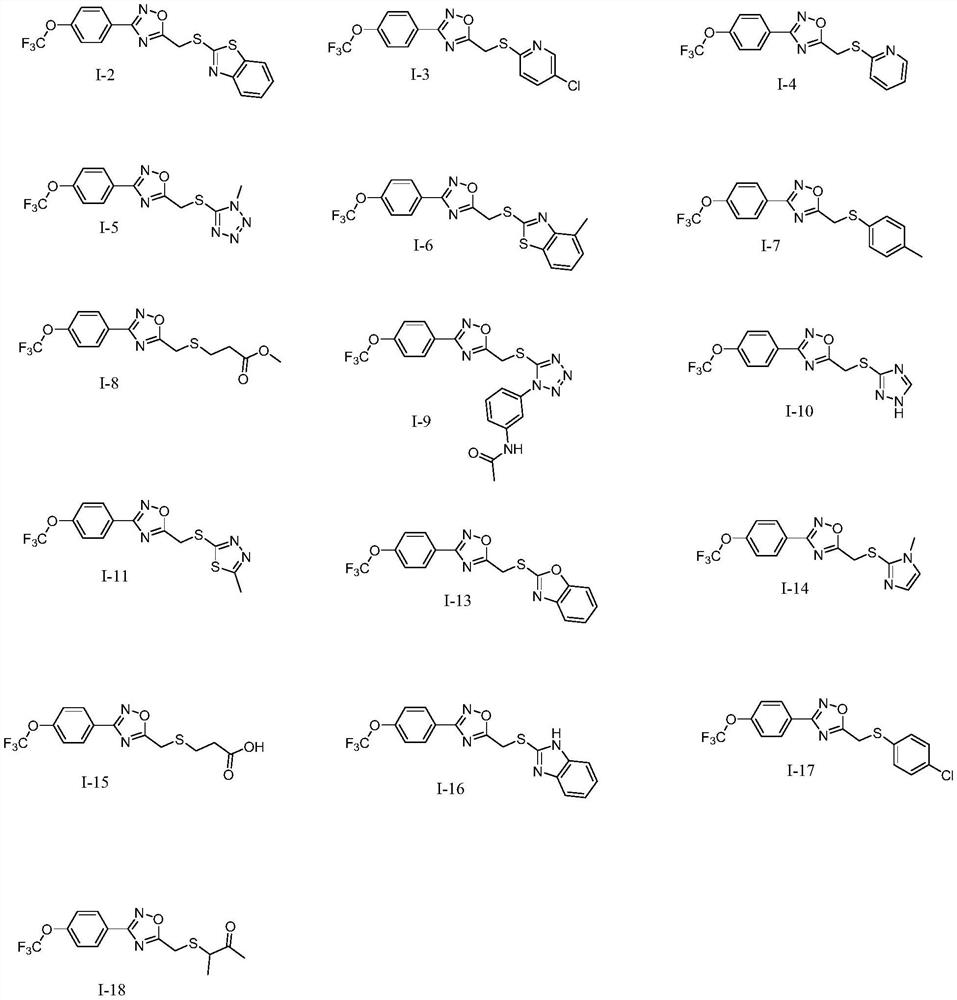
Compound with mother nucleus of 3-phenyl-1,2,4-oxadiazole as well as preparation method and application thereof
A compound, the technology of oxadiazole, which is applied in the field of medicine, can solve the problems of immunosuppression and lack of specificity, and achieve the effect of reducing inflammatory damage, simple preparation method, and improving the inflammatory microenvironment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1 synthetic compound III
[0094] Weigh hydroxylamine hydrochloride (3.9g, 0.06mol) in reaction bottle, add ethanol 70ml to it successively, sodium bicarbonate (4.8g, 0.06mol), add 4-(trifluoromethoxy) benzonitrile (5.8ml, 0.04mol), reflux reaction at 75°C for 4h, and the disappearance of raw materials was monitored by TLC. The solvent was removed under reduced pressure, extracted with ethyl acetate, washed with water, and the organic phase was collected and dried over anhydrous sodium sulfate. Silica gel column purification, the solvent was removed under reduced pressure to obtain 5.7 g of white solid, yield: 64.8%. 1 H NMR (300MHz, DMSO-d 6 )δ9.78(s,1H),7.87–7.68(m,2H),7.38(dq,J=7.9,1.1Hz,2H),5.92(s,2H). 13 C NMR (75MHz, DMSO-d 6 )δ150.28, 149.09, 133.14, 127.82, 122.21, 121.15. HRMS (ESI) calcd for C 8 h 7 f 3 N 2 o 2 [M+H] + 221.0532, found 221.0535. HPLC (10%-100% methanol in water), t R = 11.22 min, >98.17%.
Embodiment 2
[0095] Embodiment 2 synthetic compound IV
[0096] Weigh compound III (4.4g, 0.02mol) in the reaction flask, add acetone 50mL, potassium carbonate (3g, 0.02mol) to it, add chloroacetyl chloride (3.25ml, 0.04mol) dropwise, reflux at 55°C for 8h, TLC monitored disappearance of starting material. The solvent was removed under reduced pressure, tetrahydrofuran was added, the reaction was refluxed at 70°C, and the disappearance of the starting material was monitored by TLC. The solvent was removed under reduced pressure, extracted with ethyl acetate, washed with water, and the organic phase was collected and dried over anhydrous sodium sulfate. Silica gel column purification, the solvent was removed under reduced pressure. 2.3 g of light yellow oil was obtained with a yield of 41.4%. 1 H NMR (300MHz, Chloroform-d) δ8.33–8.04 (m, 2H), 7.36 (dp, J=7.9, 1.1Hz, 2H), 4.78 (s, 2H). 13 C NMR (75MHz, Chloroform-d) δ174.65, 167.88, 151.54, 129.29, 124.72, 122.07, 121.17, 33.29. HRMS (ES...
Embodiment 3
[0097] Embodiment 3 synthetic compound I-1
[0098] 5-(((1H-imidazol-2-yl)thio)methyl)-3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole (I-1)
[0099] Taking compound I-1 as an example, weigh compound IV (200mg, 0.72mmol) in a reaction flask, add tetrahydrofuran 10mL, potassium carbonate (160mg, 1.16mmol), and then add 1H-imidazole-2-thiol (100mg , 1 mmol), reacted overnight at room temperature, and TLC monitored the disappearance of the starting material. The reaction solution was sequentially extracted with ethyl acetate, washed with water, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. Purify with a silica gel column to obtain 203 mg, yield: 82.5%.
[0100]5-(((1H-imidazol-2-yl)thio)methyl)-3-(4-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole (I-1) .White solid, yield 82.5%, m.p.152.1-153.0℃. 1 H NMR (300MHz, DMSO-d6) δ12.48(s,1H), 8.13–8.05(m,2H), 7.60–7.53(m,2H), 7.09(s,2H), 4.60(s,2H). 13 C NMR (75MHz, DMSO-d6) δ178.17, 167.21, 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com