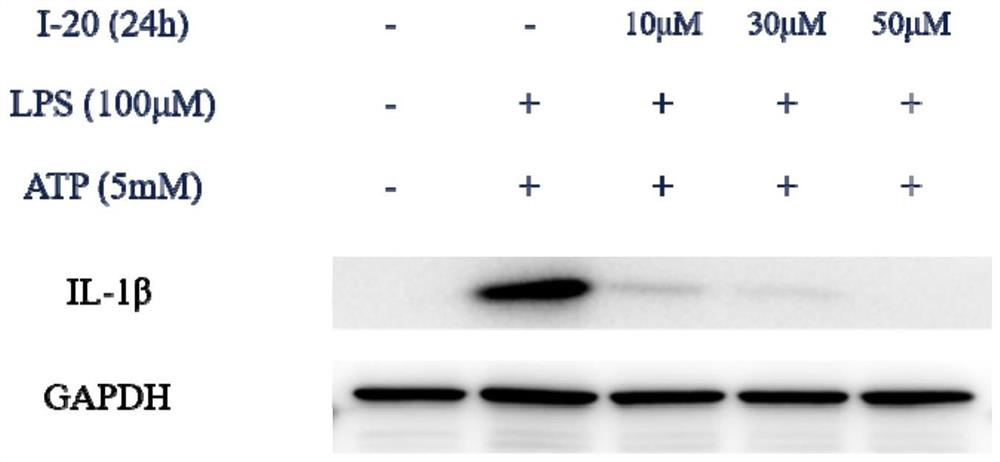
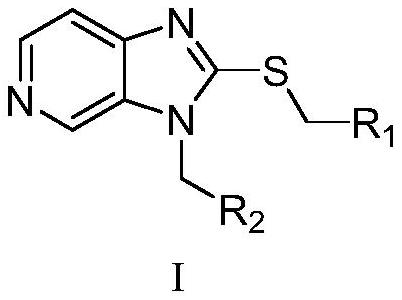
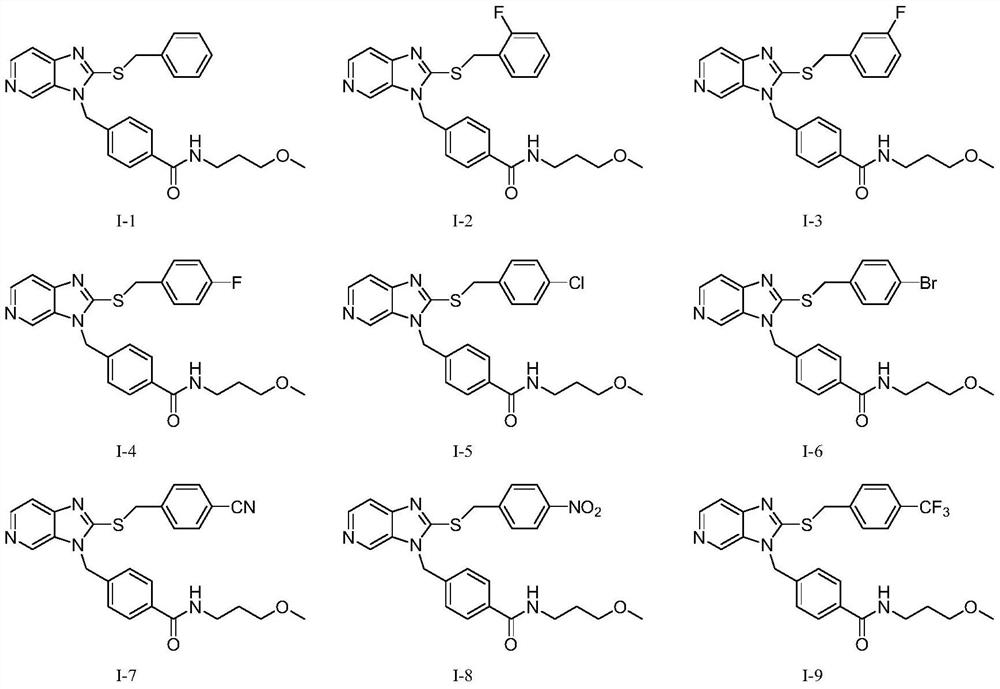
Compound with pyridoimidazole as parent nucleus as well as preparation method and application of compound
A technique for pyridimidazole and compounds, applied in the field of compounds whose mother core is pyridimidazole and its preparation, can solve the problems of large side effects and failure to become drugs, etc., reduce inflammatory damage, improve inflammatory microenvironment, and have simple preparation methods Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1 synthetic compound III
[0074] Weigh 3,4-diaminopyridine (8g, 0.0733mol) into the reaction flask, add 60ml of absolute ethanol and carbon disulfide (15.9ml, 0.264mol) to it in sequence, and react under reflux at 80°C for 4h. TLC monitors that the raw materials disappear. The reaction solution was cooled to room temperature, suction filtered, and the filter cake was washed with ethyl acetate, and dried to obtain 10.2 g of off-white solid, yield: 92.0%. 1 H NMR (300MHz, DMSO-d6) δ12.87(s, 2H), 8.37(d, J=1.0Hz, 1H), 8.23(d, J=5.4Hz, 1H), 7.16(dd, J=5.4, 1.0Hz,1H).
Embodiment 2
[0075] Embodiment 2 synthetic compound IV-1
[0076] 2-(Benzylthio)-3-hydro-imidazo[4,5-c]pyridine (IV-1)
[0077] Weigh compound III (500mg, 3.31mmol) into the reaction flask, add 5ml of N,N-dimethylformamide, anhydrous sodium carbonate (701.04mg, 6.61mmol) to it successively, heat and react at 40°C for 1h, then slowly drop Benzyl bromide (412 μl, 3.47 mmol) was added, and the reaction was continued for 4 h, and the disappearance of the starting material was monitored by TLC. The reaction solution was cooled to room temperature, poured into a separatory funnel, diluted with a large amount of water, extracted with n-butanol, washed with saturated sodium chloride, dried over anhydrous sodium sulfate, concentrated under reduced pressure to remove the solvent, and used a dichloromethane / methanol column Separation and purification by chromatography gave 519.6 mg of white solid, yield: 65.11%.
[0078] 2-(Benzylthio)-3-hydro-imidazo[4,5-c]pyridine (IV-1). White solid, yield: 65.1...
Embodiment 3
[0079] Embodiment 3 synthetic compound IV-2
[0080] 2-((2-fluorobenzyl)thio)-3-hydrogen-imidazo[4,5-c]pyridine (IV-2). The synthesis method is the same as in Example 2, and compound III (500mg, 3.31mmol ), anhydrous sodium carbonate (701.04mg, 6.61mmol), and 2-fluorobenzyl bromide (419μl, 3.47mmol) were used as raw materials to obtain 527.6mg of a white solid with a yield of 61.52%. 1 H NMR (300MHz, DMSO-d6) δ13.18(s, 1H), 8.77(s, 1H), 8.18(d, J=5.7Hz, 1H), 7.58(td, J=7.7, 1.8Hz, 1H) ,7.50(d,J=4.9Hz,1H),7.39–7.29(m,1H),7.27–7.18(m,1H),7.15(td,J=7.4,1.3Hz,1H),4.64(s,2H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com