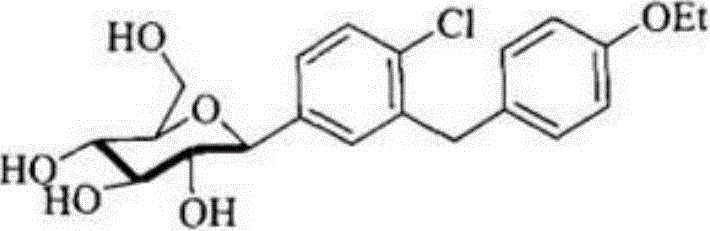
Preparation method for Dapagliflozin
A compound and halogenated hydrocarbon technology, applied in the field of preparation of dapagliflozin, can solve the problems of no industrial production value, low utilization rate of raw materials, high production cost, etc., to avoid sulfonate genotoxic impurities and post-processing Cumbersome, Inexpensive Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
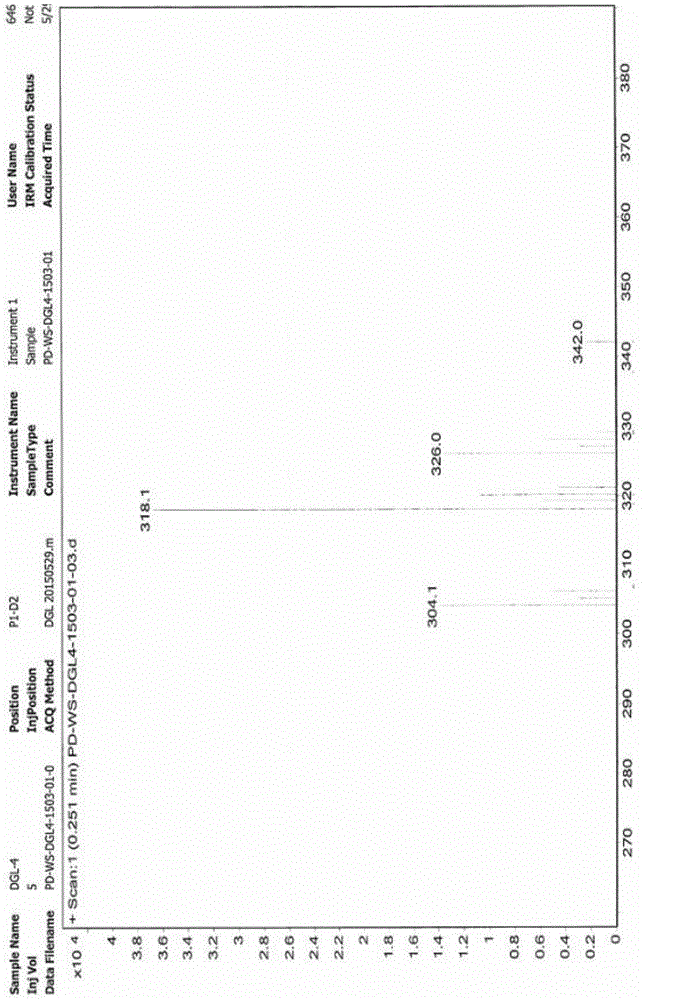
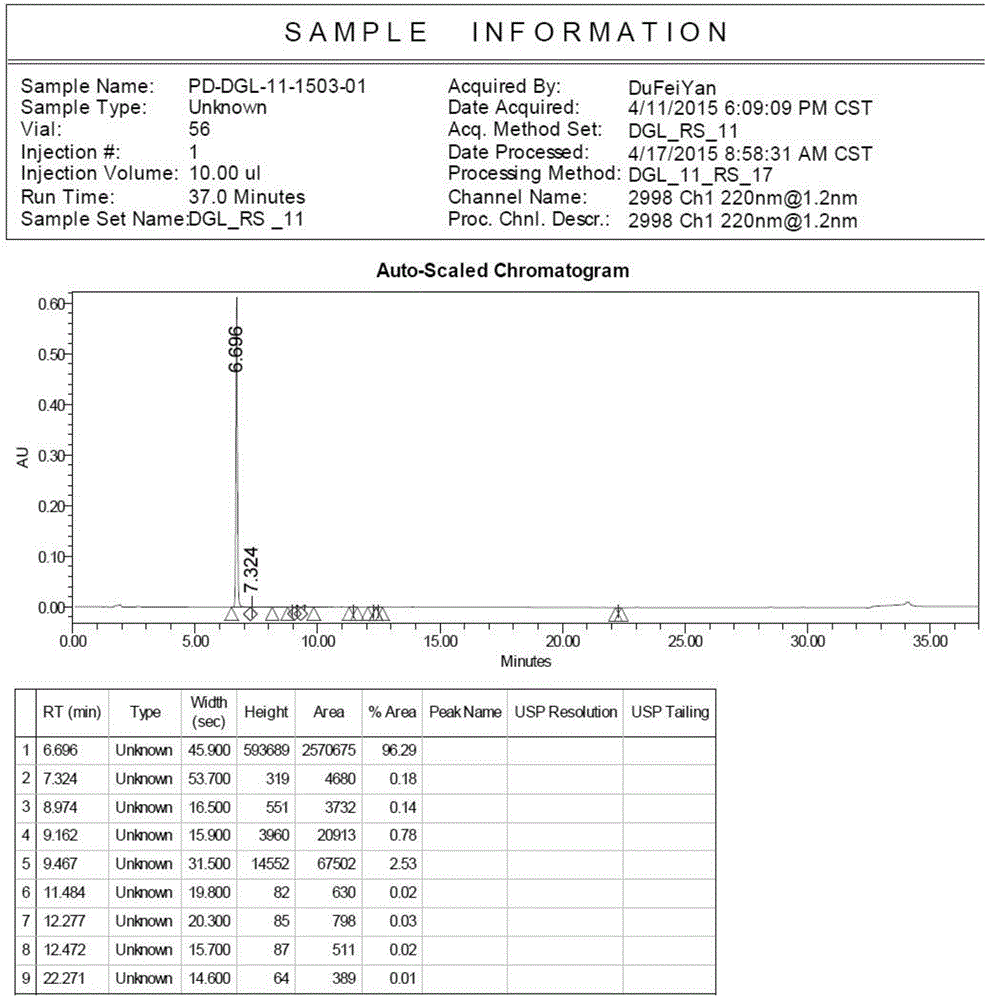
Image
Examples
Embodiment 1
[0049] The preparation method of dapagliflozin in the present embodiment comprises the following steps:
[0050] (1) Add 500mL of dichloromethane to the reaction flask, lower the temperature to -10°C under continuous nitrogen flow, and add 80g of AlCl to the reaction flask 3 , Add 0.5 mol of compound 2 and a mixed solution of 0.5 mol of phenetole and 100 mL of dichloromethane into the reaction bottle below 0°C, and keep it warm at 0-5°C until the reaction is complete as detected by TLC after dropping;
[0051] (2) Drop 106g of 1,1,3,3-tetramethyldisiloxane into the reaction flask of step (1) below 5°C, and react at room temperature until the reaction of compound 3 detected by TLC is complete; Add 250mL of 1moL / L hydrochloric acid dropwise in the bottle to quench the reaction, let stand for stratification, wash the organic layer with water, let stand for stratification again, evaporate the organic layer to obtain an oil, add ethanol to the obtained oil to crystallize, filter an...
Embodiment 2
[0059] The preparation method of dapagliflozin in the present embodiment comprises the following steps:
[0060] (1) Add 500mL of 1,1,2,2-tetrachloroethane into the reaction flask, lower the temperature to -10°C under continuous nitrogen flow, and add 80g of AlCl to the reaction flask 3 , Add 0.5 mol of compound 2 and a mixed solution of 0.5 mol of phenetole and 100 mL of dichloromethane into the reaction bottle below 0°C, and after the dropping, keep it warm at -10 to 0°C until the reaction is detected by TLC;
[0061] (2) Drop 158g of triisopropylsilane into the reaction flask of step (1) below 5°C, and react at room temperature until the reaction of compound 3 detected by TLC is complete; add 250mL of 1moL / L hydrochloric acid dropwise into the reaction flask below 5°C Quenching the reaction, standing for layering, washing the organic layer with water, then standing for layering, evaporating the organic layer to obtain an oily substance, adding ethanol crystals to the obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com