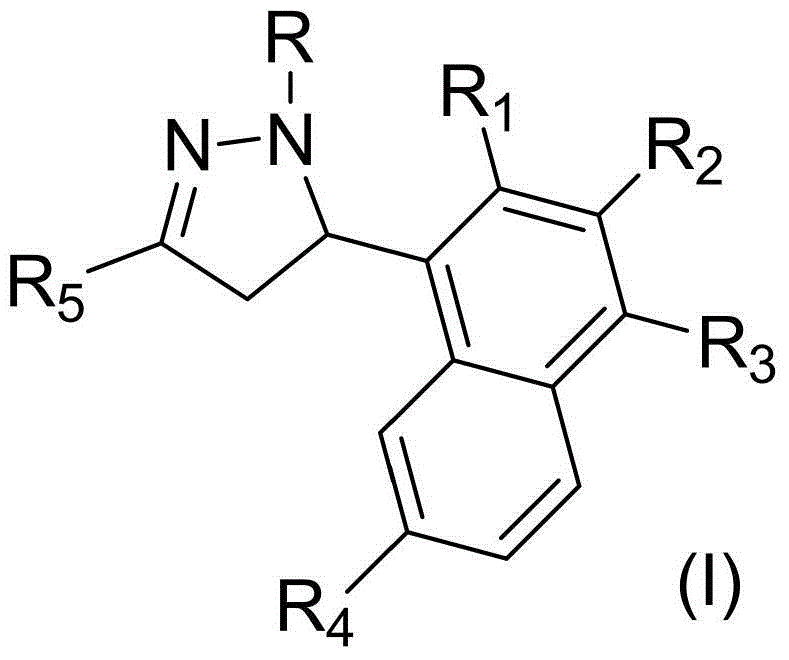
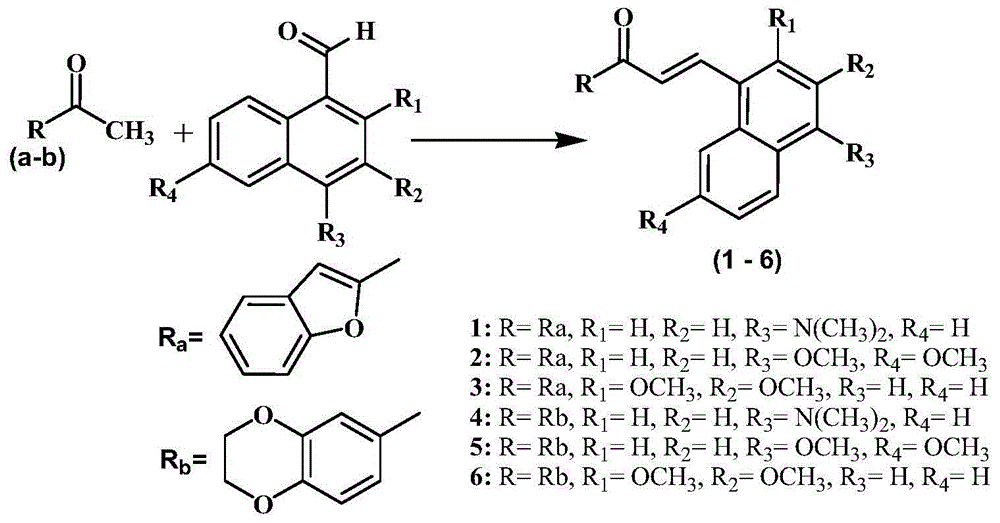
Pyrazoline derivative and application of pyrazoline derivative as tyrosinase inhibitor
A technology of pyrazoline and derivatives, which is applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., to achieve the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Compound 1-(2,3-dihydro-benzo[1,4]dioxin-6-yl)-3-(4-dimethylamino-naphthalen-1-yl)-propenone ( 4) Synthesis of:
[0042] 1-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)ethanone (1.78 g, 10 mml) was added to 4-dimethylamino-1-naphthalene Aldehyde (1.99g, 10mml) in ethanol solution (15ml). 50% NaOH solution was added dropwise to the reaction mixture to make it alkaline (PH=10), and the reaction mixture was stirred at 27°C for 8 hours. TLC monitored the completion of the reaction. The reaction solution was poured into 50 ml of hydrochloric acid ice-water mixture (1 ml of concentrated hydrochloric acid). Extracted with ethyl acetate (50ml), washed with water (150ml), dried over anhydrous magnesium sulfate, concentrated in a rotary evaporator to obtain light yellow powder 1-(2,3-dihydro-benzo[1,4]diox Heterocyclohexen-6-yl)-3-(4-dimethylamino-naphthalen-1-yl)-propenone (4) 1.76 g, yield 49%.
[0043] 1 H NMR (500MHz, CDCl 3 )δ: 7.78(d, J=8.5Hz, H), 7.66(d, J=7.0Hz, H), 7.56(d...
Embodiment 2
[0044] Example 2. Compound 3-benzofuran-2-yl-5-(4-dimethylamino-naphthalene-1-yl)-4,5-dihydro-pyrazole-1-thioformic acid amide (1a )Synthesis:
[0045] 2-Acetylbenzofuran (1.60 g, 10 mml) was added to a solution (15 ml) of 4-dimethylamine-1-naphthaldehyde (1.99 g, 10 mml) in ethanol. 50% NaOH solution was added dropwise to the reaction mixture to make it alkaline (PH=10), and the reaction mixture was stirred at 27°C for 8 hours. TLC monitored the completion of the reaction. The reaction solution was poured into 50 ml of hydrochloric acid ice-water mixture (1 ml of concentrated hydrochloric acid). It was extracted with ethyl acetate (50ml), washed with water (150ml), dried over anhydrous magnesium sulfate, and concentrated by a rotary evaporator to obtain 1.70g of chalcone (1) with a yield of 50%.
[0046] Thiosemicarbazide (0.55 g, 6 mmol) was added to a solution of chalcone (1) (1.70 g, 5 mmol) in ethanol (15 ml). 50% NaOH solution was added dropwise to the reaction mixtu...
Embodiment 3
[0048] Example 3. Compound 3-benzofuran-2-yl-5-(4,7-dimethoxynaphthalene-1-yl)-4,5-dihydro-pyrazole-1-thioformic acid amide ( Synthesis of 2a):
[0049] Using 4,6-dimethoxy-1-naphthylaldehyde instead of 4-dimethylamine-1-naphthaldehyde, the others were the same as in Example 2 to obtain white powder pyrazoline (2a) with a yield of 39%.
[0050] 1 H NMR (500MHz, CDCl 3 )δ:8.87(s,D 2 O exchangeable, 2H, NH 2 ),7.89-7.29(m,J=8.0Hz, 8H),7.16(s,1H),6.92(d,J=8.0Hz,1H),5.57-5.50(dd,J=11.5Hz,1H),4.22 -4.19(dd, J=11.5Hz, 1H), 3.85(s, 6H), 3.47-3.34(dd, J=17.5Hz, 1H); Mass Spectrum(ESI) m / z: 432.52[M+H]+ ;Microanalysis Calculated for C 24 h 21 N 3 o 3 S(431.51), C, 66.80%; H, 4.91%; N, 9.74%; S, 7.43%. Found C: 66.97%, H: 4.98%, N: 9.73%, S: 7.41%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com