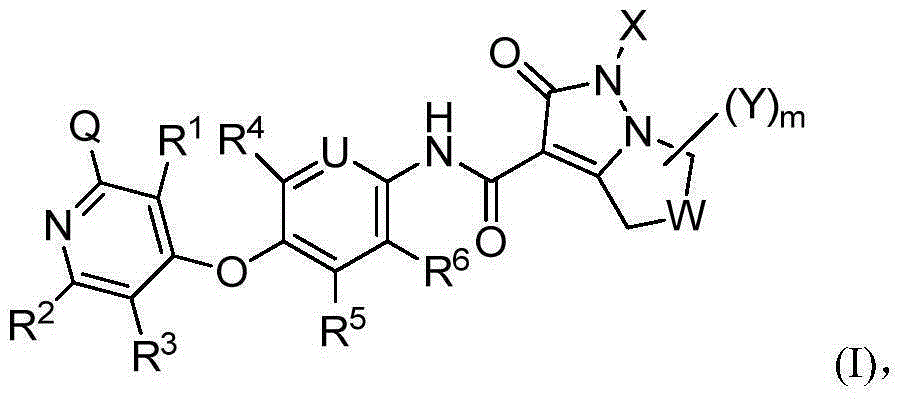
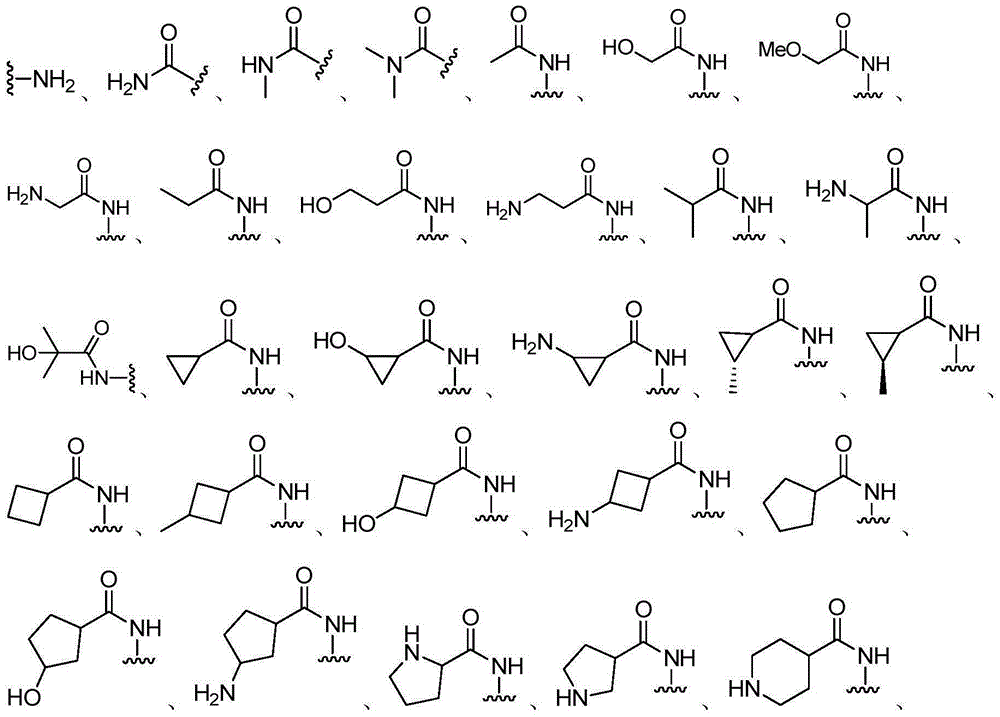
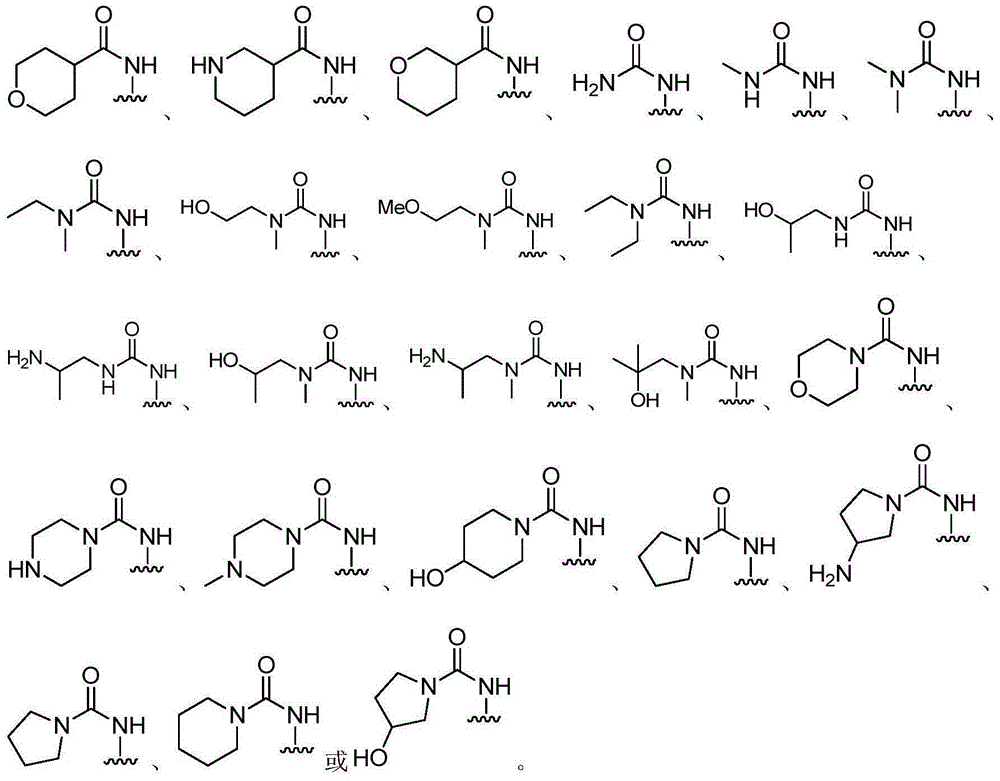
Bicyclic pyrazolone compound, and use method and applications thereof
A technology of compounds and nitrogen oxides, applied in the field of compounds that inhibit intercellular or intracellular signal response, human hyperproliferative diseases, and pharmaceutical compositions containing the compounds of the present invention, can solve problems such as poor prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0335] Example 1N-(4-((2-aminopyridin-4-yl)oxy)phenyl)-2-oxo-1-phenyl-2,4,5,6-tetrahydro-1H-pyrrolo [1,2-b]pyrazole-3- Formamide
[0336]
[0337] Step 1) 4-Chloro-N'-phenylbutyric acid hydrazide
[0338] Will contain phenylhydrazine (16.0g, 148.0mmol) and 10% Na 2 CO 3A mixture of aqueous solution (250 mL) and dichloromethane (250 mL) was cooled to 0 °C, and 4-chlorobutyryl chloride (20.9 g, 148.0 mmol) was added thereto by syringe. The reaction solution was returned to room temperature, stirred overnight, and diluted with dichloromethane (150 mL). The organic phase was separated, and the resulting organic phase was washed successively with 2M hydrochloric acid (300 mL x 3), brine (150 mL), then dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting residue was recrystallized from a mixture of ethyl acetate / n-hexane (50 mL / 100 mL) to obtain the title compound (14.7 g, 47%) as a light-colored solid.
[0339] LC-MS(ESI,...
Embodiment 2
[0368] Example 2N-(4-((2-(cyclopropylcarboxamido)pyridin-4-yl)oxy)phenyl)-2-oxo-1-phenyl-2,4,5,6-tetra Hydrogen-1H-pyrrolo[1,2-b] Pyrazole-3-carboxamide
[0369]
[0370] To the N-(4-((2-aminopyridin-4-yl)oxy)phenyl)-2-oxo-1-phenyl-2,4,5,6-tetrahydro-1H-pyrrolo [1,2-b]Pyrazole-3-carboxamide (150mg, 0.35mmol) and pyridine (1.4mL) in acetonitrile (2mL) were added cyclopropanoyl chloride (110mg, 1.05mmol) and DMAP (128.3mg, 1.05 mmol) in acetonitrile (1.4 mL). The reaction solution was heated and stirred at 60°C overnight, then cooled to room temperature, and diluted with dichloromethane / water (10 mL / 15 mL). The separated organic phase was washed with water (6 mL x 3), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting residue was recrystallized from ethyl acetate / petroleum ether (8 mL, v / v=1 / 3) to give the title compound (109 mg, 60%) as a pale solid.
[0371] LC-MS(ESI,pos,ion)m / z:496.3[M+H] + ;
[0372] Q-TOF(ESI,p...
Embodiment 3
[0375] Example 3N-(4-((2-acetylaminopyridin-4-yl)oxy)phenyl)-2-oxo-1-phenyl-2,4,5,6-tetrahydro-1H-pyrrole And[1,2-b]pyrazole -3-Carboxamide
[0376]
[0377] To the N-(4-((2-aminopyridin-4-yl)oxy)phenyl)-2-oxo-1-phenyl-2,4,5,6-tetrahydro-1H-pyrrolo To a solution of [1,2-b]pyrazole-3-carboxamide (100 mg, 0.23 mmol) in acetic anhydride (2 mL) was added triethylamine (142 mg, 1.4 mmol). After the reaction solution was stirred overnight at 30°C, it was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (100% ethyl acetate) to give the title compound (38 mg, 35%) as a light-colored solid.
[0378] LC-MS(ESI,pos,ion)m / z:470.2[M+H] + ;
[0379] Q-TOF(ESI,pos,ion)m / z:470.1827[M+H] + ;
[0380] 1 H NMR (600MHz, CDCl 3 )δ(ppm):10.22(s,1H),8.27(s,1H),8.08(d,J=5.8Hz,1H),7.86(s,1H),7.75(d,J=8.9Hz,2H) ,7.54(t,J=7.9Hz,2H),7.44(d,J=7.5Hz,2H),7.40(t,J=7.4Hz,1H),7.07(d,J=8.9Hz,2H),6.56 (dd, J=5.8Hz, 2.2Hz, 1H), 3.73(t, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com