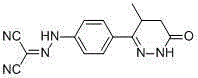
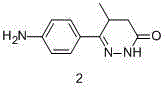
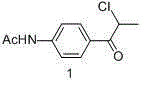
Synthesis process of levosimendan
A compound, the technology of chloropropionyl chloride, which is applied in organic chemistry and other fields, can solve the problems of long synthetic route and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add acetanilide and aluminum trichloride in a molar ratio of 1:3 to a 1L flask in turn, add dichloromethane, the volume ratio of the mass of acetanilide to dichloromethane is 1:3, and stir to dissolve. Add 2-chloropropionyl chloride dropwise into the reactor, the molar ratio of 2-chloropropionyl chloride to acetanilide is 1.2:1, and the dropwise addition is completed within 1 hour. The reaction was incubated at 25°C for 3 hours, cooled, poured into ice water, filtered and washed with deionized water three times, and recrystallized from ethanol to obtain compound 1 with a yield of 84.2%.
[0024] Add metal sodium to a 1L flask, add ethanol, the mass ratio of the volume of ethanol to metal sodium is 40:1, slowly add diethyl malonate, diethyl malonate dropwise under ice bath cooling after the reaction is completed The molar ratio of ester to sodium metal is 1:2, the dropwise addition is completed within 1h, and the reaction is stirred for 2h. Compound 1 was added into the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com