A class of depsipphenolic acid compounds derived from marine fungi and their application in the treatment of type 2 diabetes
A technology of depacids and marine fungi, applied in the directions of microorganisms, metabolic diseases, organic chemistry, etc., can solve the problems of elevated blood sugar, defects, insufficient insulin secretion, etc., and achieves the effects of low cost, simple method and good market prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Marine fungus Meyerozyma sp.HZ-Y of the present invention 2 It is isolated from the root of the mangrove plant Candela chinensis in the sea area of Huizhou, Guangdong. The separation method refers to the conventional separation method in this field. After isolation, the marine fungus Meyerozyma sp.HZ-Y 2 It was deposited in the China Center for Type Culture Collection on April 6, 2015. The deposit address is Wuhan University, Wuhan, China, and the deposit number is CCTCC NO: M 2015203.
[0029] From the marine fungus Meyerozyma sp.HZ-Y 2 Separate and obtain depsipated phenolic acid compounds in the fermented liquid of the method, concrete steps are as follows:
[0030] S1. Seed cultivation:
[0031] S11. Preparation of seed medium: 200g of potatoes, 20g of glucose, 1L of tap water, evenly distributed in five 500mL Erlenmeyer flasks, and extinguished at 121°C for 30 minutes.
[0032] S12. Cultivation of seeds: the marine fungus Meyerozyma sp.HZ-Y 2 The strain of t...
Embodiment 2
[0039] The compound in embodiment 1 is carried out structural analysis test, obtains following physical and chemical property data:
[0040] Compound 1: light yellow powder, melting point 146-147°C (the thermometer is not corrected), EI-MS (m / z): 288 [M] + .
[0041] Compound 2: Pale yellow needle-like crystals, melting point 165-166°C (the thermometer is not corrected), EI-MS (m / z): 300[M] + .
[0042] Compound 3: white powder, melting point 147-148°C (the thermometer is not corrected), EI-MS (m / z): 302[M] + .
[0043]Compound 4: light yellow powder, melting point 143-144°C (the thermometer is not corrected), EI-MS (m / z): 316[M] + .
[0044] Compound 5: Pale yellow oil, EI-MS (m / z): 302[M] + .
[0045] Compound 6: Pale yellow needle-like crystals, melting point 169-170°C (the thermometer is not corrected), EI-MS (m / z): 314[M] + .
[0046] The NMR data of compounds 1-3 are shown in Table 1, and the NMR data of 4-5 are shown in Table 2.
[0047] NMR data (100MHz / 400MH...
Embodiment 3
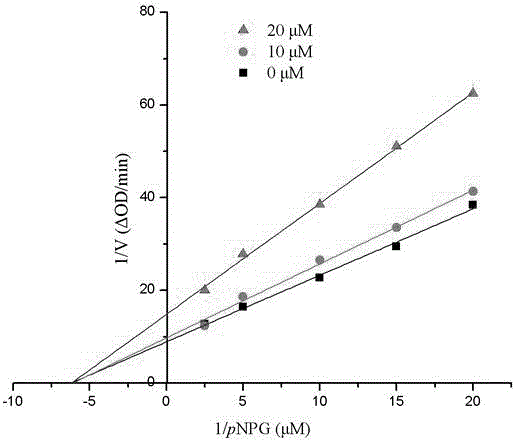
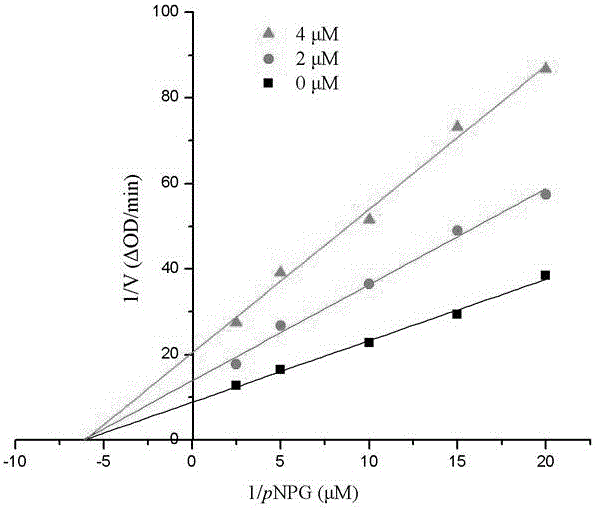
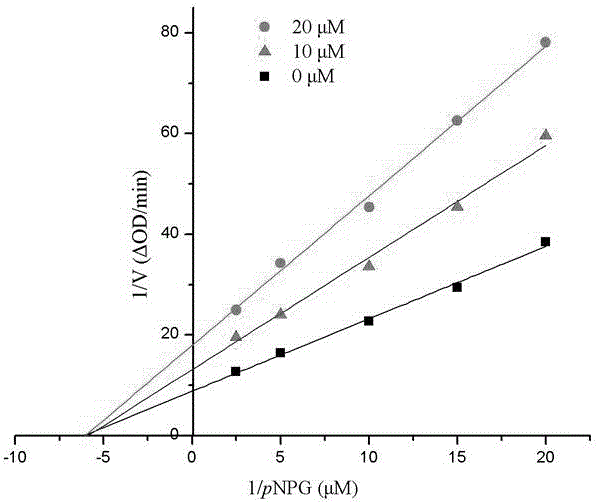
[0055] Compound 1-6 in embodiment 1 is carried out α-glucosidase inhibition test:
[0056] Using p-nitrophenol-α-glucoside (pNPG) as a substrate, it was carried out in 0.01M phosphate buffer (pH=7.0). pNPG was enzymatically hydrolyzed into p-nitrophenol by α-glucosidase, and the enzyme activity was calculated by measuring the change of its absorbance at a wavelength of 400nm with a UV-visible spectrophotometer. Both the sample and the positive control (acarbose) were made into DMSO solution (both 10 μmol / mL), and the enzyme and substrate were made into a suitable concentration solution with 0.01M phosphate buffer. 1 mL of the initial reaction system contained 0.1 unit enzyme, 20 μL Substrate, 20 μL DMSO. Take an appropriate amount of enzyme solution, add the blank DMSO solution or sample DMSO solution, mix well, keep the temperature at 37°C for 20 minutes, add the substrate, mix well, and immediately detect the change value of the absorbance of the system within 1min at a wav...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com