Aryl dihydronaphthalene lignan compound as well as preparation method and application thereof
The technology of aryl dihydronaphthalene lignans and compounds is applied in the field of medicine, and can solve the problems such as the obstruction of chemotherapy of cancer patients, and achieve the effects of simple extraction and separation method, strong selective inhibitory activity, and small toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
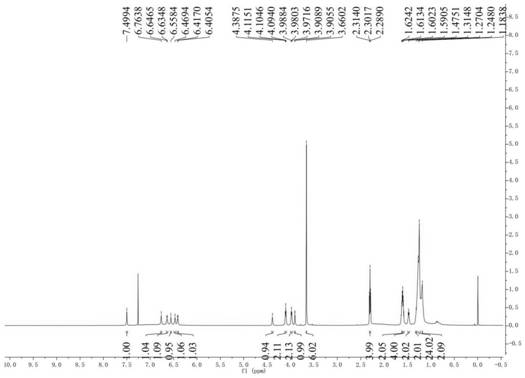
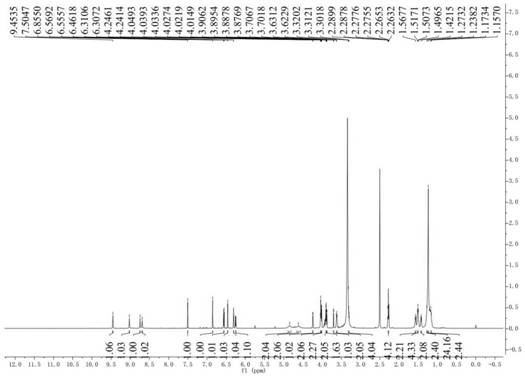
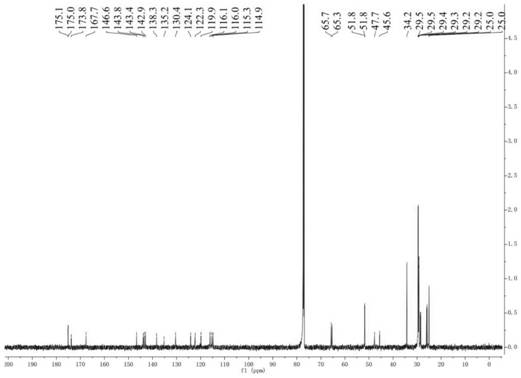
[0031] Preparation and structure identification of compounds 1 and 2
[0032] A method for extracting, separating and preparing aryl dihydronaphtalignins with antitumor activity, comprising the following steps:
[0033] (1) Take Drynaria rhizomes, extract 3-5 times at room temperature with 95% methanol and then concentrate to obtain extract. After the extract is dissolved in methanol, extract with equal volume of petroleum ether and concentrate to obtain petroleum ether layer extract and methanol layer extract; the methanol layer extract is miscible with water to form turbidity, and is extracted and concentrated by equal volumes of petroleum ether and ethyl acetate mixed solution to obtain a medium polar layer extract;
[0034] (2) The medium polar layer extract is subjected to 200-300 mesh silica gel column chromatography, using petroleum ether: ethyl acetate = (100: 0 ~ 50: 1), dichloromethane: ethyl acetate = 50: 1 ~ 10 : 1. Dichloromethane: Methanol = 20:1 ~ 2:1 ratio of ...
Embodiment 2
[0050] Embodiment 2: the synthesis of compound 1
[0051] Dissolve 12-hydroxylauric acid (1.0g, 4.6mmol) in 40mL of methanol, slowly drop into 25 drops of sulfuric acid solution, heat and reflux for 4h, then cool the reaction to room temperature, and spin the methanol out under vacuum, then wash the mixture with ethyl acetate (20 mL), washed with distilled water and saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain a crude product. The crude product was separated and purified by semi-preparative HPLC to obtain 0.871 g of white oily product 12-hydroxylaurate methyl ester, yield 82%, wherein the mobile phase of semi-preparative HPLC was methanol:water (0.15% TFA) volume ratio was 70:30. 1 H NMR (CDCl 3 ,600MHz)δ3.62(s,3H),3.58(t,J=6.7Hz,2H),2.26(t,J=7.5Hz,2H),1.58-1.55(m,2H),1.53-1.50(m ,2H),1.31-1.29(m,2H),1.26-1.20(m,12H). 13 C NMR (150MHz, CDCl 3 )δ174.5,62.9,51.5,34.2,32.8,29.6,29.5×3,29.3,29.2,25.8,25.0; ESI-MS m / z 253.2...
Embodiment 3
[0053] Embodiment 3: the synthesis of compound 2
[0054] Dissolve glyceryl 12-caffeoyloxylaurate (20mg, 0.04mmol) in 1.5mL acetone, stir in an ice-water bath for 5 minutes, then slowly drop FeCl 3 ·6H 2O (120 mg, 0.44 mmol) in 100 μL of aqueous solution, stirred at 5°C for 1.5 hours, and reacted in an ice-water bath for 38 hours. After the solvent acetone was evaporated in vacuo, the mixture was diluted with ethyl acetate (10 mL), and diluted with distilled water and saturated sodium chloride. The solution was washed, dried over anhydrous sodium sulfate, and then concentrated in vacuo to obtain the crude coupling product. The crude product was separated on a Sephadex LH-20 gel column with equal volumes of methanol and dichloromethane as the mobile phase, and further purified by semi-preparative HPLC to obtain a brown oily product. 2 (1.8mg, 10%), wherein the mobile phase of semi-preparative HPLC is acetonitrile: water volume ratio is 55:45. HR-ESIMS m / z 925.45306 ([M+Na] +...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com