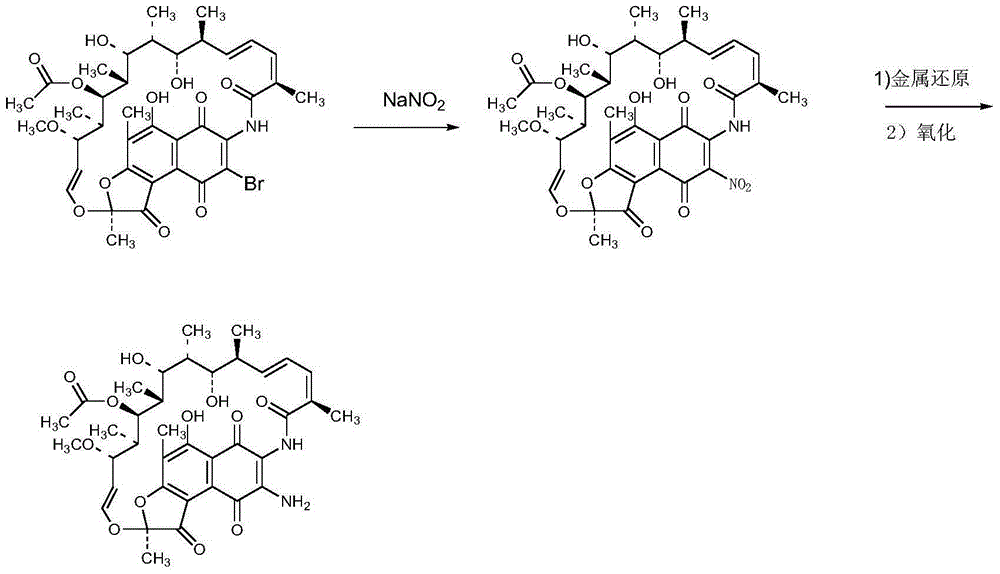
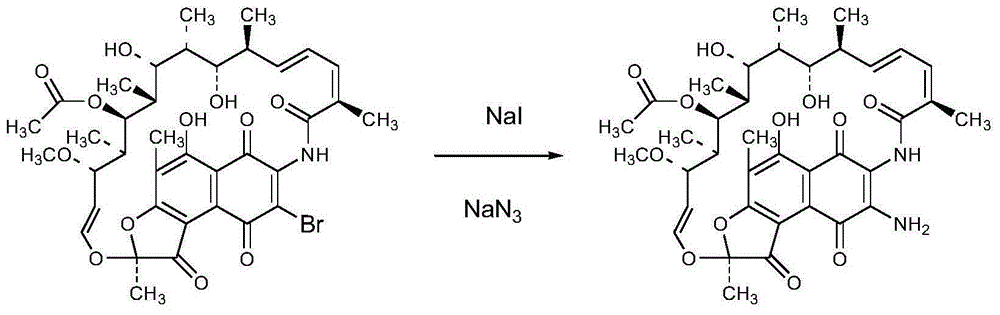
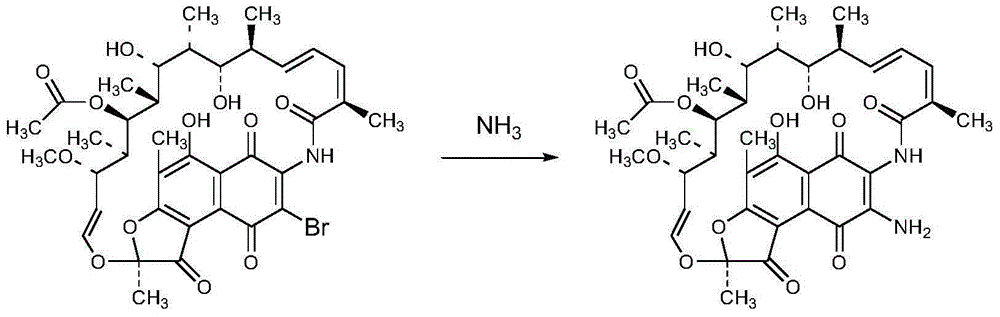
Preparation method of 3-amino-rifamycin S
A rifamycin and amino technology, applied in the field of preparation of 3-amino-rifamycin S, can solve the problems of inability to realize industrial industrialization, increase environmental protection costs, complicated operations, etc. The effect of reducing the generation of waste liquid and the simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of 3-amino-rifamycin S
[0029] Add 200g of 3-bromo-rifamycin S into a three-necked flask, add 2L of 1,4-dioxane, stir to dissolve, control the temperature at 32±3°C, and dropwise add an ammonia-methanol solution with a mass percentage of ammonia of 8%. The mixed solution of 472ml and 2L of 1,4-dioxane, after the dropwise addition, send a sample for HPLC monitoring, after the reaction is completed, concentrate the 1,4-dioxane until the flow is cut off, add ethylene glycol monomethyl ether Heat to 60°C and stir for 30 minutes, cool to about 25±5°C, then maintain the system temperature at 5±5°C and stir for 1 hour, filter, and vacuum-dry the filter cake at 40°C for 4 hours to obtain 3-amino-rifamycin S 170g, yield 92.9%, purity 99.1%, MS: M+1=718.8, M+Na=733.5.
Embodiment 2
[0030] Example 2 Preparation of 3-amino-rifamycin S
[0031] Add 20g of 3-bromo-rifamycin S into a three-necked flask, add 200ml of tetrahydrofuran, stir to dissolve, control the temperature at 32±3°C, add dropwise a mixture of 47ml of ammonia methanol solution with a mass percentage of 8% ammonia and 200ml of tetrahydrofuran After the dropwise addition is completed, send samples to HPLC for monitoring. After the reaction is completed, concentrate the tetrahydrofuran until the flow is cut off. Add ethylene glycol monomethyl ether and heat to 60°C for 30 minutes, then cool to 25±5°C, and then maintain the system Stir at 5±5°C for 1 hour, filter, and vacuum-dry the filter cake at 40°C for 4 hours to obtain 16.2 g of 3-amino-rifamycin S with a yield of 88.5% and a purity of 99.2%.
Embodiment 3
[0032] Example 3 Preparation of 3-amino-rifamycin S
[0033] Add 10g of 3-bromo-rifamycin S into a three-necked flask, add 50ml of methanol, stir to dissolve, control the temperature at 32±3°C, add dropwise 44ml of ammonia-methanol solution with a mass percentage of ammonia of 8%, after the dropwise addition , send samples to HPLC monitoring, after the reaction is completed, concentrate the tetrahydrofuran until the flow is cut off, add glycol monomethyl ether, heat to 60°C and stir for 30 minutes, cool to 25±5°C, and then keep the system temperature at 5±5°C and stir After filtering for 1 hour, the filter cake was vacuum-dried at 40° C. for 4 hours to obtain 8.5 g of 3-amino-rifamycin S with a yield of 92.4% and a purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com