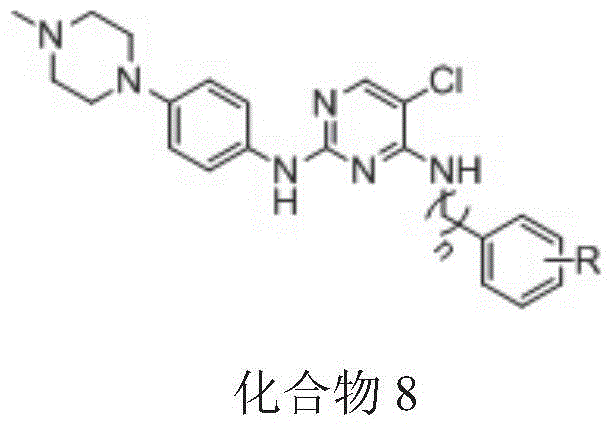
A pyrimidine egfr t790m Inhibitor and its synthesis method and application
An EGFRT790M and inhibitor technology, applied in the field of pyrimidine EGFRT790M inhibitors and their synthesis, can solve the problems of large drug side effects, stop research and development, large side effects and risks, avoid covalent binding reactions, reduce toxic side effects, Effect of strong proliferation inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
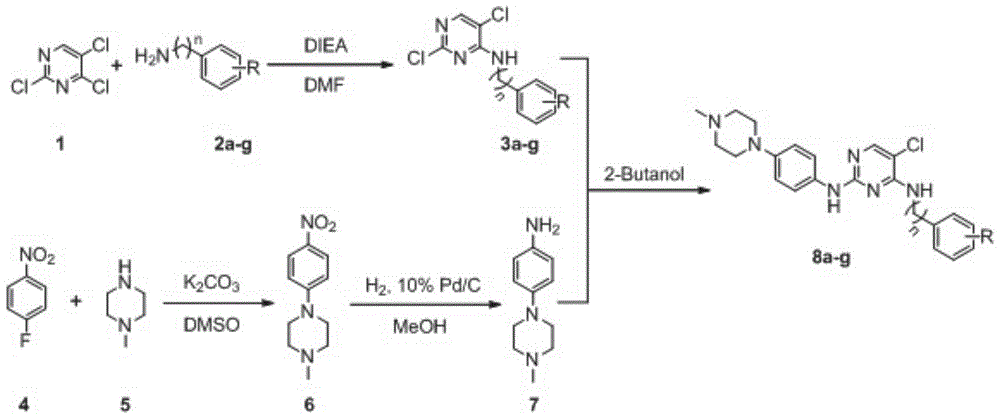
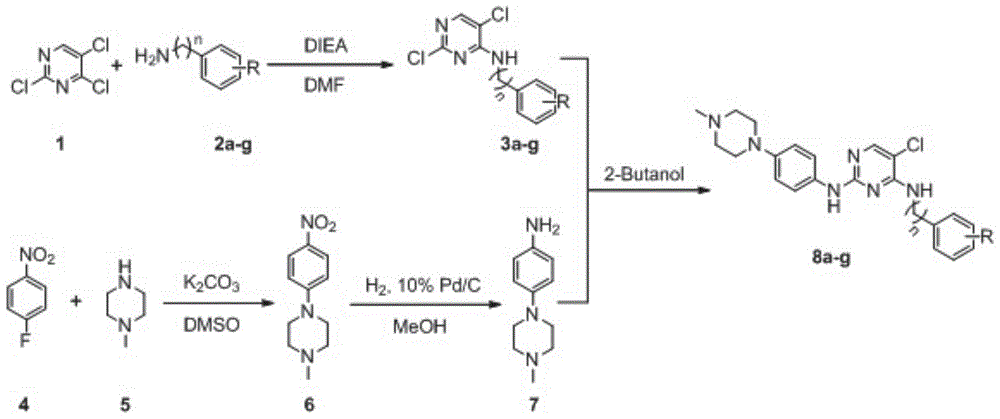
[0036] 1.1 Synthetic route
[0037] The synthesis of the target compound is based on 2,4,5-trichloropyrimidine (compound 1) as a raw material, and under DIEA basic conditions, it undergoes nucleophilic substitution with benzene (benzyl)amine (compound 2a-g) with various substituents After the reaction, 4-phenyl(benzyl)aminopyrimidine intermediates 3a-3g are obtained. At the same time, p-nitrofluorobenzene (compound 4) in potassium carbonate (K 2 CO 3 ) under weak base conditions, react with p-methylpiperazine (compound 5) to obtain compound 6, and compound 6 is reduced by hydrogen under the condition of Pd / C to obtain aniline intermediate 7. Finally, under the action of 2-butanol, intermediate 7 reacted with previously obtained 4-phenyl(benzyl)aminopyrimidine intermediates 3a-3g to obtain target compounds 8a-8g, respectively.
[0038]
[0039] 1.2 Synthesis experiment
[0040] 1.2.1 Synthesis of compounds 3a~3g (take 3a as an example)
[0041] Dissolve 1.0 g (6.66 mmol...
Embodiment 2
[0053] In vitro anti-tumor activity test
[0054] ELISA method was used to test the effect of target compound 8a-8g on EGFR T790M Inhibitory activity of protein kinases. Compounds were diluted with DMSO from stock solutions to the concentrations tested. Enzyme reaction substrate Poly(Glu, Tyr) 4:1 coats the microtiter plate and reacts at 37°C for 12-16 hours. Discard the liquid in the well. Wash the plate three times with PBST, and dry the microplate in an oven at 37°C for 1-2 hours. Add reaction buffer (50mM HEPES pH 7.4, 50mM MgCl 2 , 0.5mM MnCl 2 , 0.2mM Na 3 VO 4 , 1mMDTT) diluted ATP solution, add the compound to be tested, then add EGFR diluted with reaction buffer T790M Kinases initiate the reaction. Put it on a shaker (100 rpm) at 37°C for 1 h. The liquid in the wells was discarded, and the plate was washed three times with PBST. Add antibody PY99 diluent (dilute the antibody 1:500 with T-PBS containing 5 mg / mL BSA), and react on a shaker at 37°C for 0.5h. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com