Method for preparing charge modified lactoferrin
A technology of lactoferrin and charge modification, which is applied in the field of preparation of charge-modified lactoferrin, can solve the problems of high price, unquantitative research, and unclear curative effect, and achieve high negative conversion rate, good curative effect, and convenient use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
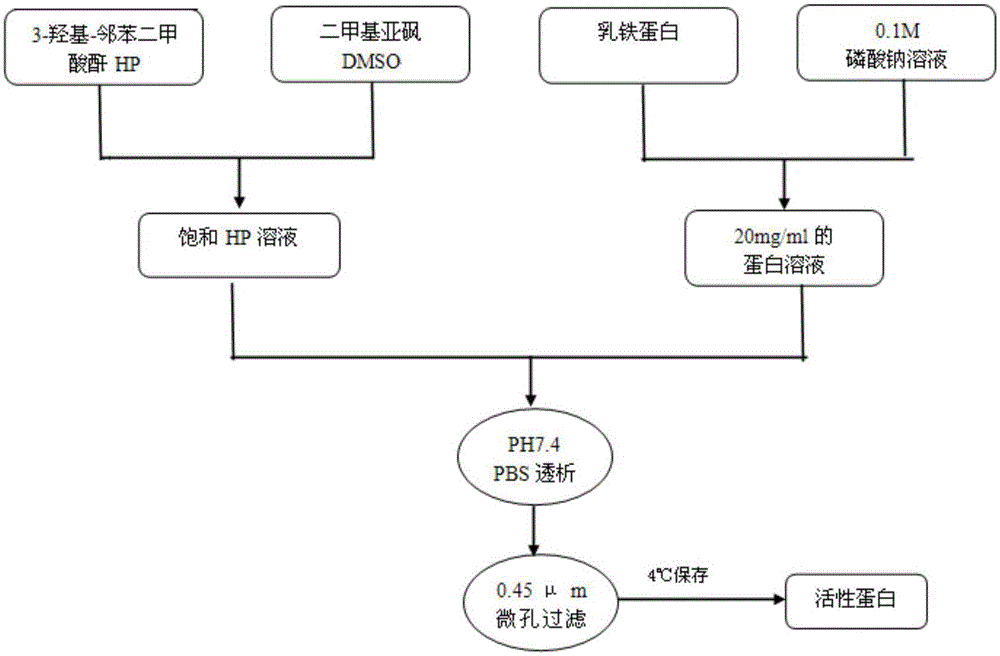
[0032] Example 1: Preparation of charge-modified lactoferrin
[0033] Put 1.65 (0.8-16.5) parts by weight of 3-hydroxy-phthalic anhydride (HP) in a 10mL small beaker, add an appropriate amount of dimethyl sulfoxide (DMSO), and stir at 200-500rpm in a magnetic stirrer, At the same time, DMSO was added dropwise to about 10 (5-100) parts by weight to prepare a saturated HP solution; under stirring at 200-500 rpm, 2 g of lactoferrin was dissolved in 100 mL of 0.1 M sodium phosphate buffer solution to prepare a 20 mg / mL solution. Protein solution; then take saturated HP solution and 20mg / mL protein solution and mix at a ratio of 1:100, stir for 15-30min to mix evenly, and adjust the pH to 10 with phosphoric acid or sodium phosphate, let it stand at 25°C for 1h, and use pH7.4 dialyzed with PBS, and sterilized by 0.45 μm membrane filtration to obtain charge-modified lactoferrin, specifically as figure 1 shown.
Embodiment 2
[0034] Embodiment 2: combination medicine
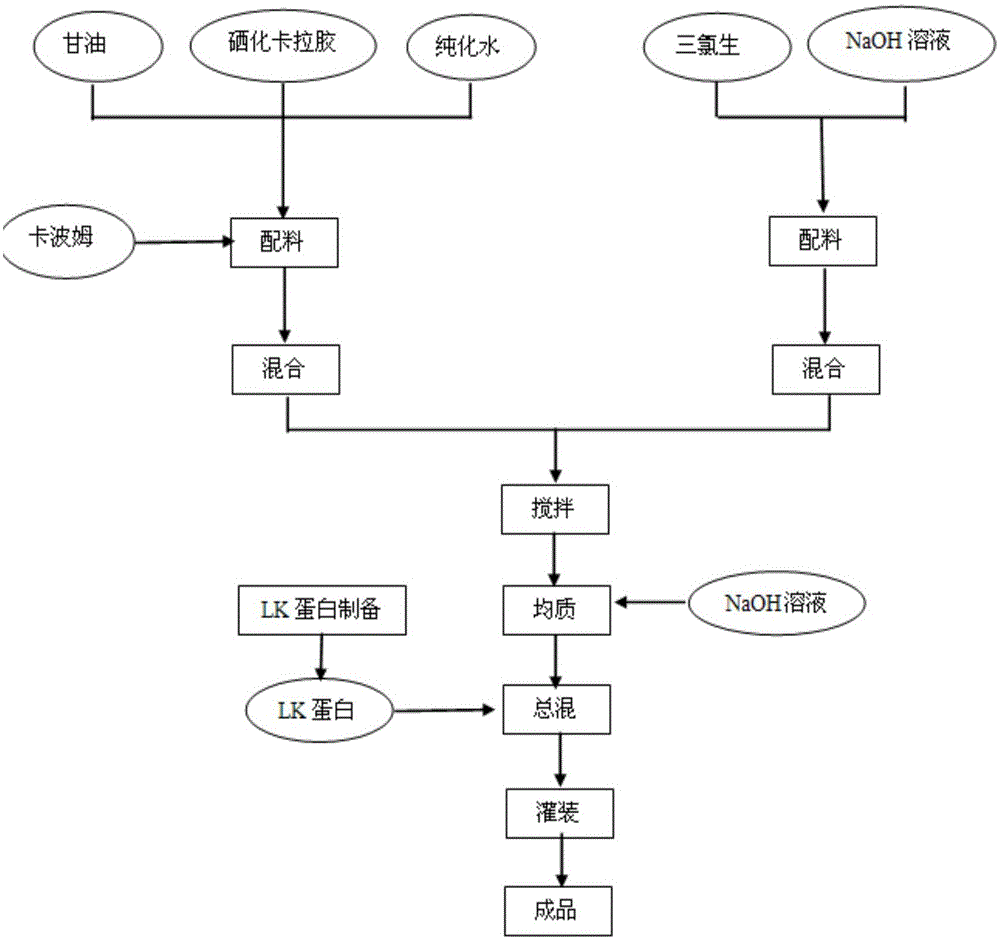
[0035] The combined medicine consists of 0.01 parts by weight of LK protein (charge-modified lactoferrin), 1.5 parts by weight of carrageenan and auxiliary materials, a total of 100 parts by weight. The auxiliary materials include 5 parts by weight of carbomer, 50 parts by weight of glycerin, 0.1 parts by weight of ethylparaben, the combined medicine is a kind of gel, suppository and dressing.
Embodiment 3
[0036] Embodiment 3: the preparation method of combination medicine
[0037] S1: Weigh each raw material according to the proportion, stir 0.5g carbomer in water at 80°C until fully swollen, add 0.15g carrageenan, stir until completely dissolved, and filter and sterilize through a 0.22μm membrane while hot to obtain 1 No. solution;
[0038]S2: Dissolve 0.01g of ethylparaben in water, then add 5g of glycerol, filter and sterilize through a 0.22μm membrane to obtain No. 2 solution;
[0039] S3: Add No. 2 solution to No. 1 solution under agitation and mix evenly, add 0.8 g of triethanolamine, adjust the pH to 5.5, and obtain No. 3 solution;
[0040] S4: Dissolve 0.001g of LK protein in an appropriate amount of water with low-speed stirring at room temperature, and filter and sterilize through a 0.22 μm membrane to obtain solution No. 4;
[0041] S5: under stirring at a certain temperature, add No. 4 solution into No. 3 solution and mix evenly, and adjust the pH to 5.5 with ster...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com