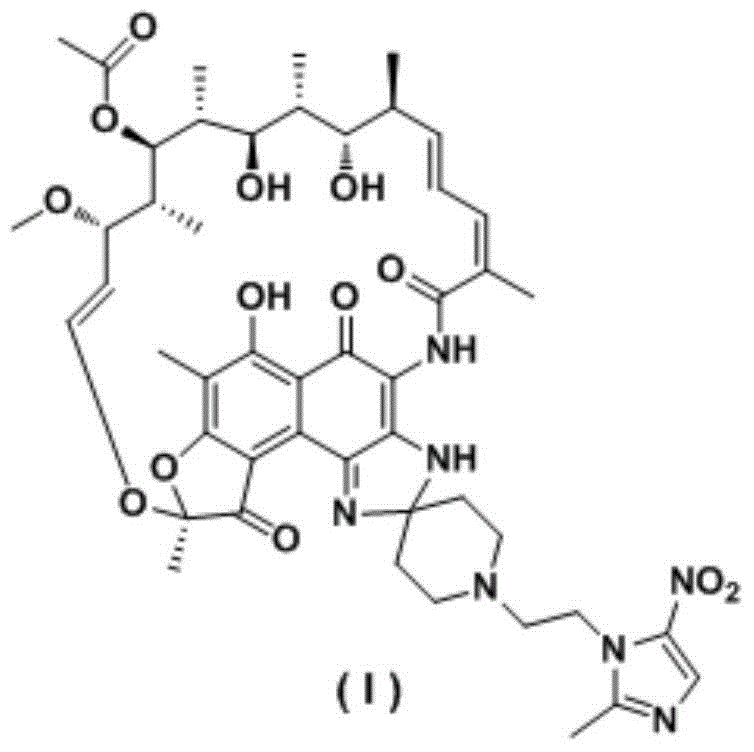
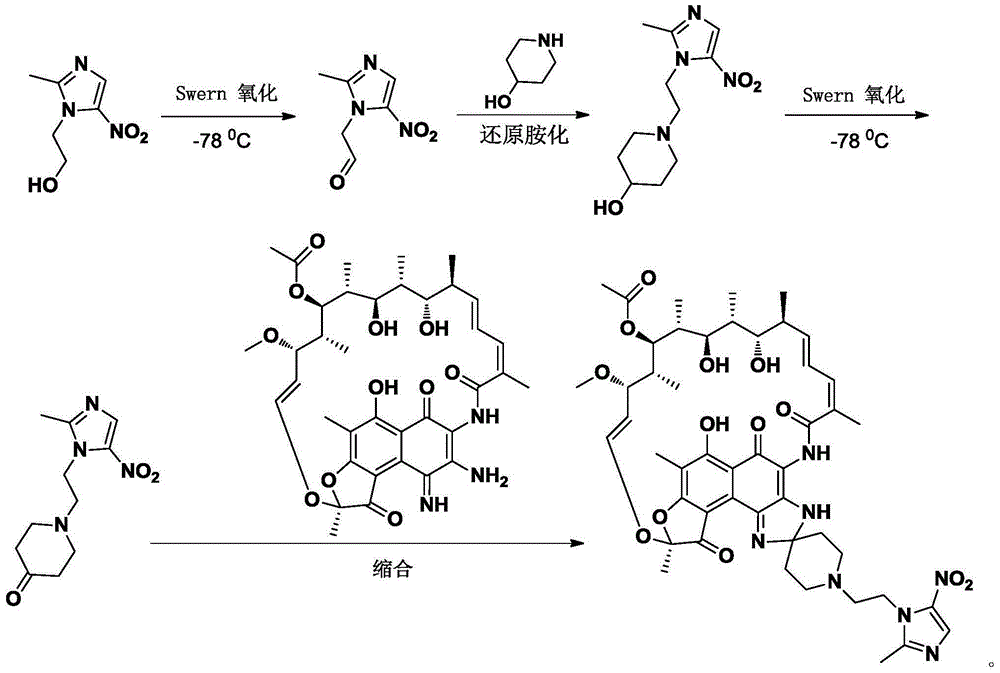
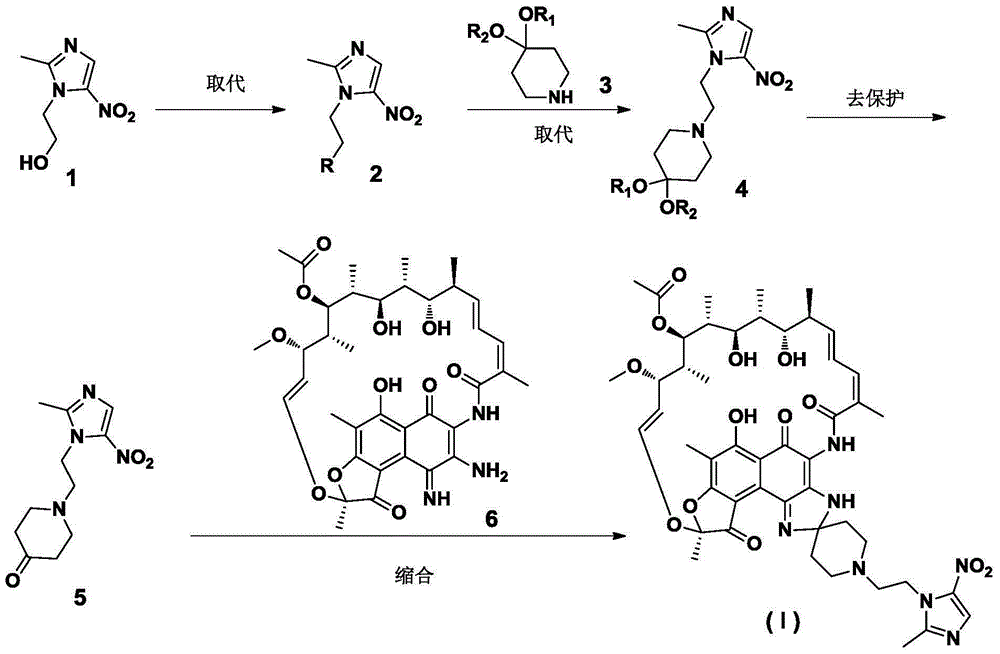
Preparation method of rifamycin-nitroiminazole coupled molecule
A technology of nitroimidazole coupling and rifamycin is applied in the field of preparation of rifamycin-nitroimidazole coupling molecules, which can solve the problems of low product yield and the like, and achieve the effect of improving product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 A kind of preparation method of rifamycin-nitroimidazole coupling molecule
[0026] Step 1: Synthesis of 2-(2-methyl-5-nitro-1hydro-imidazol-1-yl)ethyl methanesulfonate
[0027]
[0028] Add dry dichloromethane (500mL) into a 1L three-necked flask, add metronidazole (98.6g, 0.576mol), then add 4-dimethylaminopyridine (3.5g, 0.03mol) and triethylamine (120mL) . After cooling down to 0°C, methanesulfonyl chloride (54 mL, 0.692 mol) was slowly added dropwise to the reaction solution. After the dropwise addition, the reaction was slowly raised to room temperature and stirred overnight. After the reaction was complete, the solid was filtered out, and the solid was dried in vacuo to further remove residual solvent. The resulting crude product was added to 500 mL of water, stirred, filtered, and the filter cake was vacuum-dried to obtain 2-(2-methyl-5-nitro-1 hydrogen-imidazol-1-yl) ethyl methanesulfonate (139 g , yield 97.5%). Mass Spectrum (ESI, M+1): The...
Embodiment 2
[0038] Embodiment 2 A kind of preparation method of rifamycin-nitroimidazole coupling molecule
[0039] Step 1: Synthesis of 2-(2-methyl-5-nitro-1hydro-imidazol-1-yl)ethyl trifluoromethylsulfonate
[0040]
[0041] Add dry dichloromethane (500mL) into a 1L three-necked flask, add metronidazole (98.6g, 0.576mol), then add 4-dimethylaminopyridine (3.5g, 0.03mol) and triethylamine (120mL) . After cooling to -10°C, trifluoromethanesulfonic anhydride (195 g, 0.692 mol) was slowly added dropwise. After the dropwise addition, the temperature was slowly raised to room temperature and stirred overnight. After the reaction was complete, the solid was filtered out, and the filter cake was vacuum-dried. The resulting crude product was added to 500 mL of water, stirred, filtered, and the filter cake was vacuum-dried to obtain 2-(2-methyl-5-nitro-1 hydrogen-imidazol-1-yl)ethyl trifluoromethylsulfonium Ester (173 g, yield 99.1%). Mass Spectrum (ESI, M+1): theoretical 304.0, found 304....
Embodiment 3
[0050] Embodiment 3 A kind of preparation method of rifamycin-nitroimidazole coupling molecule
[0051] Step 1: Synthesis of 2-(2-methyl-5-nitro-1hydro-imidazol-1-yl)ethyl p-toluenesulfonate
[0052]
[0053] Add dry dichloromethane (500mL) into a 1L three-necked flask, add metronidazole (98.6g, 0.576mol), then add 4-dimethylaminopyridine (3.5g, 0.03mol) and triethylamine (120mL) . After cooling to 10°C, p-toluenesulfonyl chloride (132 g, 0.692 mol) was slowly added dropwise to the reaction liquid. After the dropwise addition, the temperature was slowly raised to room temperature, and stirred overnight. After the reaction was complete, the solid was filtered out, and the filter cake was vacuum-dried. The obtained crude product was added into 500 mL of water, stirred, filtered, and the filter cake was vacuum-dried to obtain the title compound (158.3 g, yield 84.5%). Mass spectrum (ESI, M+1): theoretical 326.1, found 326.1.
[0054] Step 2: Synthesis of 9-(2-(2-methyl-5-ni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com