Method for preparing hydroxy neovaleraldehyde
A technology for hydroxypivalaldehyde and formaldehyde, applied in the field of preparing hydroxypivalaldehyde, can solve the problems of low product selectivity and yield, low utilization rate of raw materials, etc. simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
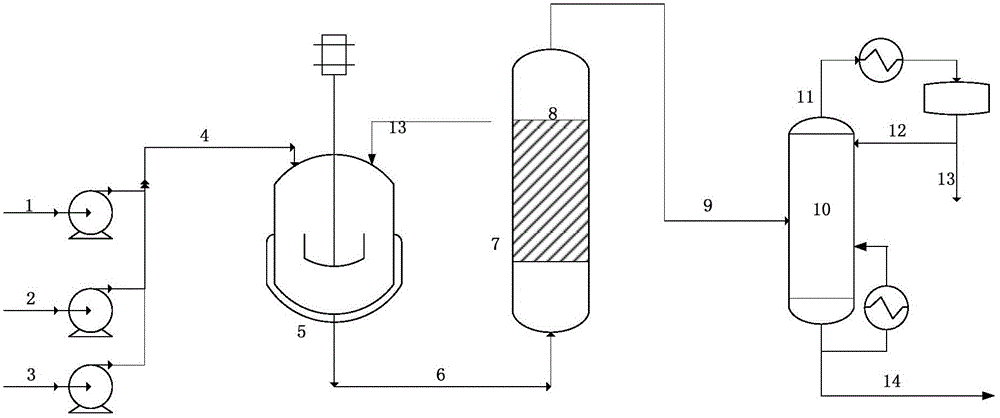
[0028] in such as figure 1 In the shown process flow, the feed molar ratio of formaldehyde and isobutyraldehyde is 1.05:1, (formaldehyde aqueous solution of about 6.6ml / min (formaldehyde mass content is 37wt%), about 7.8ml / min industrial isobutyraldehyde (isobutyraldehyde The mass content of butyraldehyde is> 98wt%)) and about 1.2ml / min trimethylamine aqueous solution (33%) are mixed in the reaction pipeline, put into a 1L stirred tank for reaction, at a reaction temperature of 75°C and a pressure of 0.2MPaG, stay for 45min Under the conditions of reaction, the material at the outlet of the reactor enters the secondary tubular reactor. The inner diameter of the tubular reactor is 25mm, and the length of the tube is 1500mm. No internal parts are added to the tube. The material direction is from bottom to top. Under the conditions of 75°C, normal pressure, and residence time of 45 minutes, the reaction produces an aqueous solution of hydroxypivalaldehyde. The composition of the...
Embodiment 2
[0030]According to the conditions and steps described in Example 1, the molar ratio of formaldehyde and isobutyraldehyde feed is 1.1:1, about 6.91ml / min of formaldehyde solution (formaldehyde mass content is 37wt%), 8.17ml / min of industrial isobutyraldehyde Aldehyde (mass content of isobutyraldehyde is > 98wt%) and trimethylamine aqueous solution (33%) of 1.3ml / min are mixed in the reaction pipeline, add in 1L stirred tank and carry out reaction, at reaction temperature 75 ℃, pressure is 0.2MPaG, After the reaction under the condition of staying for 45 minutes, the material at the outlet of the reactor enters the two-stage tubular reactor, and the structured packing is filled in the tubular reactor, and the material direction is from bottom to top. Under the condition of 45min, the reaction produces an aqueous solution of hydroxypivalaldehyde. The reaction results are: the conversion rate of isobutyraldehyde is 96.53%, and the selectivity of hydroxypivalaldehyde+neopentyl glyc...
Embodiment 3
[0032] According to the conditions and steps described in Example 1, the molar ratio of formaldehyde and isobutyraldehyde feed is 0.8:1, about 5.02ml / min formaldehyde solution (formaldehyde mass content is 37wt%), about 5.94ml / min industrial isobutyl Aldehyde (mass content of isobutyraldehyde is> 98wt%) and about 0.9ml / min sodium hydroxide aqueous solution (10%) are catalysts, mixed in the reaction pipeline, enter the reactor, the reaction conditions are reaction temperature 85 ℃, pressure 0.3MPaG , stay for 30 minutes, the material at the outlet of the reactor enters the two-stage tubular reactor, no internal parts are added in the tubular reactor, and the direction of the material is from bottom to top. At a reaction temperature of 85 ° C, the pressure is normal pressure, and the residence time is 30 minutes. Under certain conditions, the reaction produces an aqueous solution of hydroxypivalaldehyde. The reaction results are: the conversion rate of isobutyraldehyde is 94.33%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com