Preparation method for iron and zinc glycine complex
A technology of iron-zinc glycine and glycine, which is applied in the field of preparation of glycine complexes, can solve problems such as unfavorable industrial production, product purity needs to be improved, and small product output, so as to reduce production costs and labor intensity, improve the production and operation environment of employees, Avoid the effect of noise and dust
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
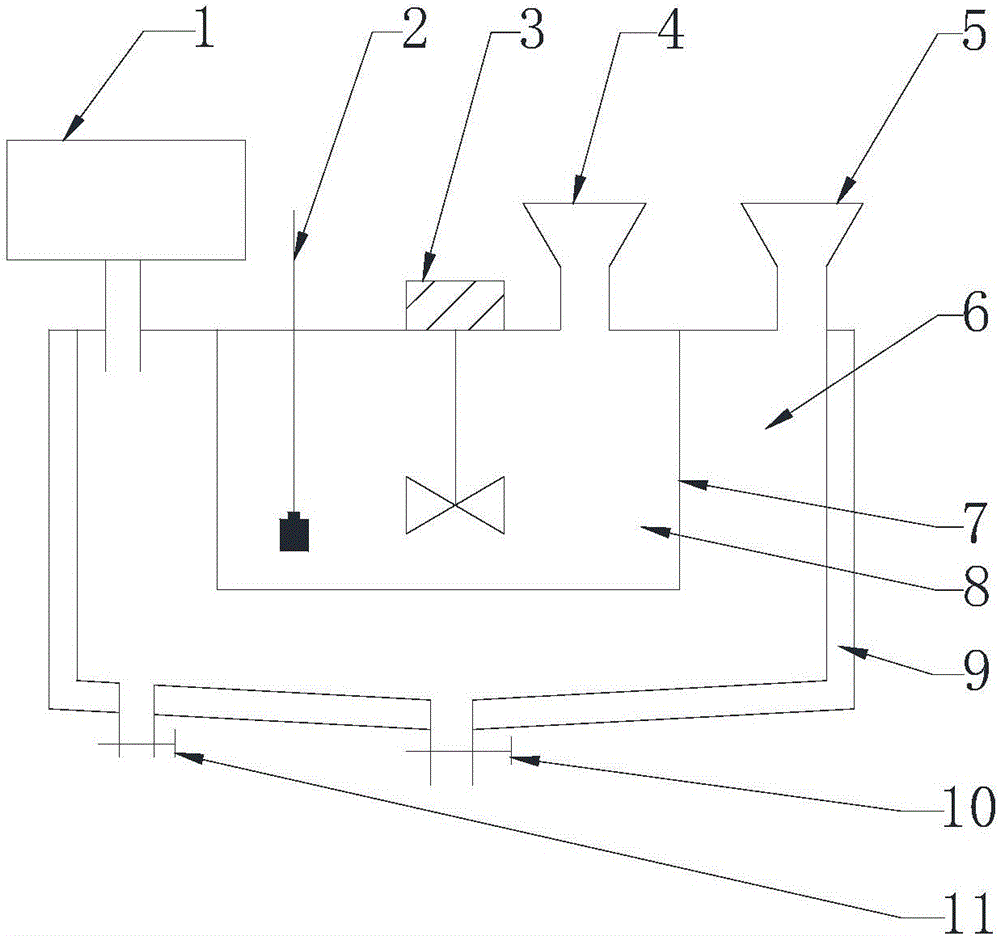
[0055] A kind of preparation method of the iron-zinc glycine complex that can be used for animal feed additive of the present invention, comprises the following steps:
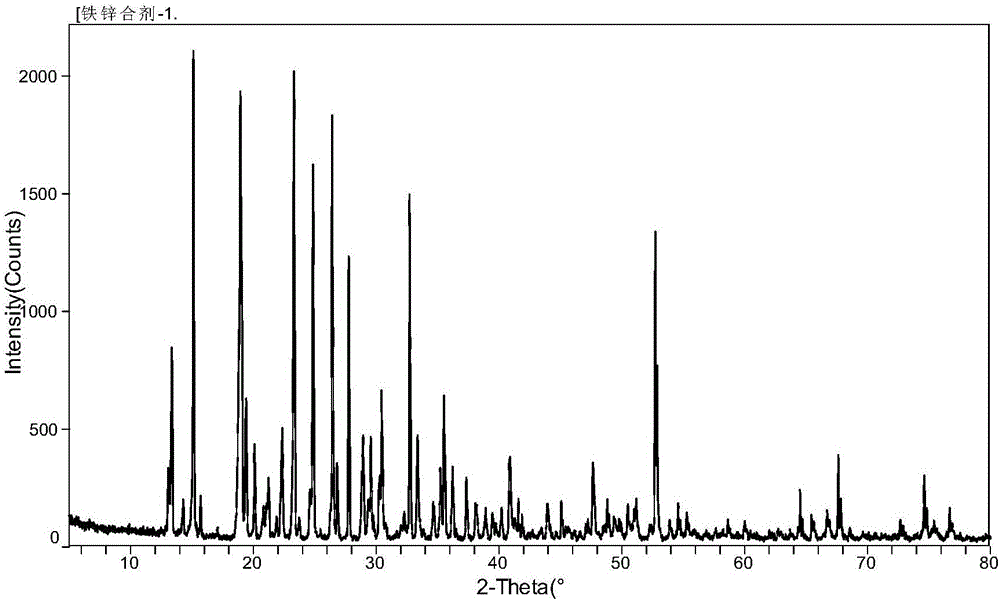
[0056] Taking by weighing 50.5g purity is 98% glycine, 143.5g purity is 97% ferrous sulfate heptahydrate, 29.6g purity is 97% zinc sulfate heptahydrate and drops into reactor, zinc element and heptahydrate in zinc sulfate heptahydrate The molar ratio of the iron element in the water ferrous sulfate is controlled to be about 1:5, and the ratio of the molar number of glycine to the total molar number of the aforementioned zinc element and iron element is about 1.1:1, then stir evenly, add 300mL water, and adjust the reaction temperature 20°C, adjust the reaction pH to 3.0, stir for 15 minutes, cool and stir for crystallization, then centrifuge, wash the crystals, vacuum dry, pulverize and sieve to obtain 183.0 g of iron-zinc glycine complex.
[0057] After detection and analysis, the Fe-zinc complex of glycine o...
Embodiment 2
[0061] A kind of preparation method of the iron-zinc glycine complex that can be used for animal feed additive of the present invention, comprises the following steps:
[0062]Taking by weighing 38.3kg purity is 98% glycine, 56.1kg purity is 91% ferrous sulfate monohydrate, 38.1kg purity is 94% zinc sulfate monohydrate and drops into reactor, zinc element and a The molar ratio of the iron element in the ferrous sulfate water is controlled to be about 2:3, the ratio of the molar number of glycine to the total molar number of the aforementioned zinc element and iron element is about 1:1, then stir evenly, add water-ethyl acetate to mix Solvent (water: ethyl acetate volume ratio is 1: 2) 120kg, adjust reaction temperature to be 45 ℃, adjust reaction pH value to be 4.5, stir reaction for 45 minutes, centrifuge after cooling and stirring crystallization, wash crystallization, vacuum drying, Pulverize and sieve to obtain 153.7 kg of glycine iron-zinc complex.
[0063] After detecti...
Embodiment 3
[0065] A kind of preparation method of the iron-zinc glycine complex that can be used for animal feed additive of the present invention, comprises the following steps:
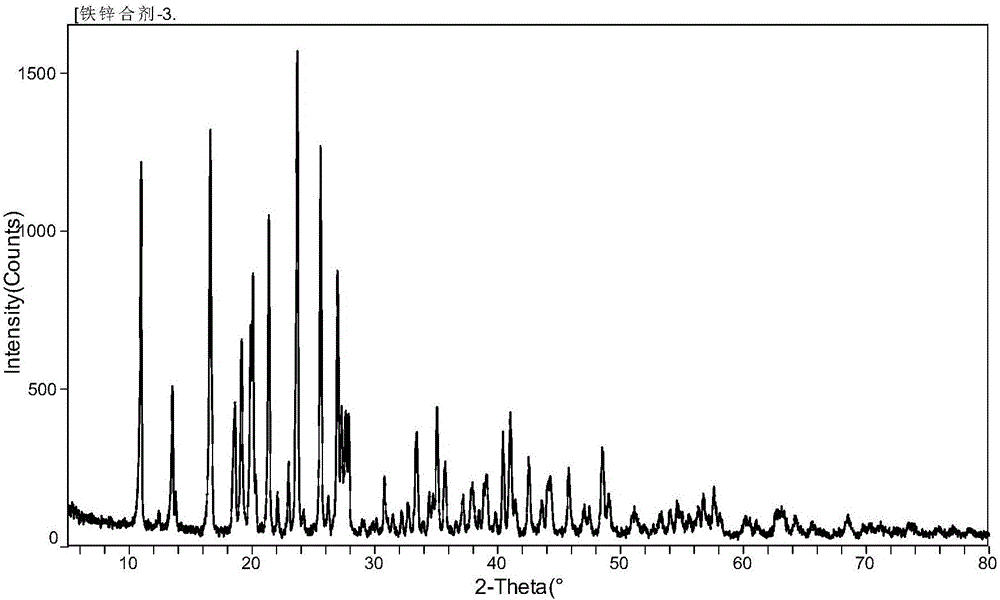
[0066] Taking by weighing 48.3kg purity is 98% glycine, 14.0kg purity is 91% ferrous chloride, 70.1kg purity is 97% zinc chloride and drops in the reactor, zinc element and ferrous chloride in the zinc chloride The mol ratio of iron element in the middle is controlled to be about 5: 1, and the ratio of the molar number of glycine to the total molar number of aforementioned zinc element and iron element is about 1.05: 1, then stir well, add water-ethanol mixed solvent (water: ethanol volume The ratio is 5:1) 200Kg, the reaction temperature is adjusted to 35°C, the reaction pH value is adjusted to 5.0, the reaction is stirred for 50 minutes, cooled, stirred and crystallized, centrifuged, washed, dried in vacuum, crushed and sieved to obtain Iron Zinc Glycinate Complex 166.1kg.
[0067] After detection and analy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com