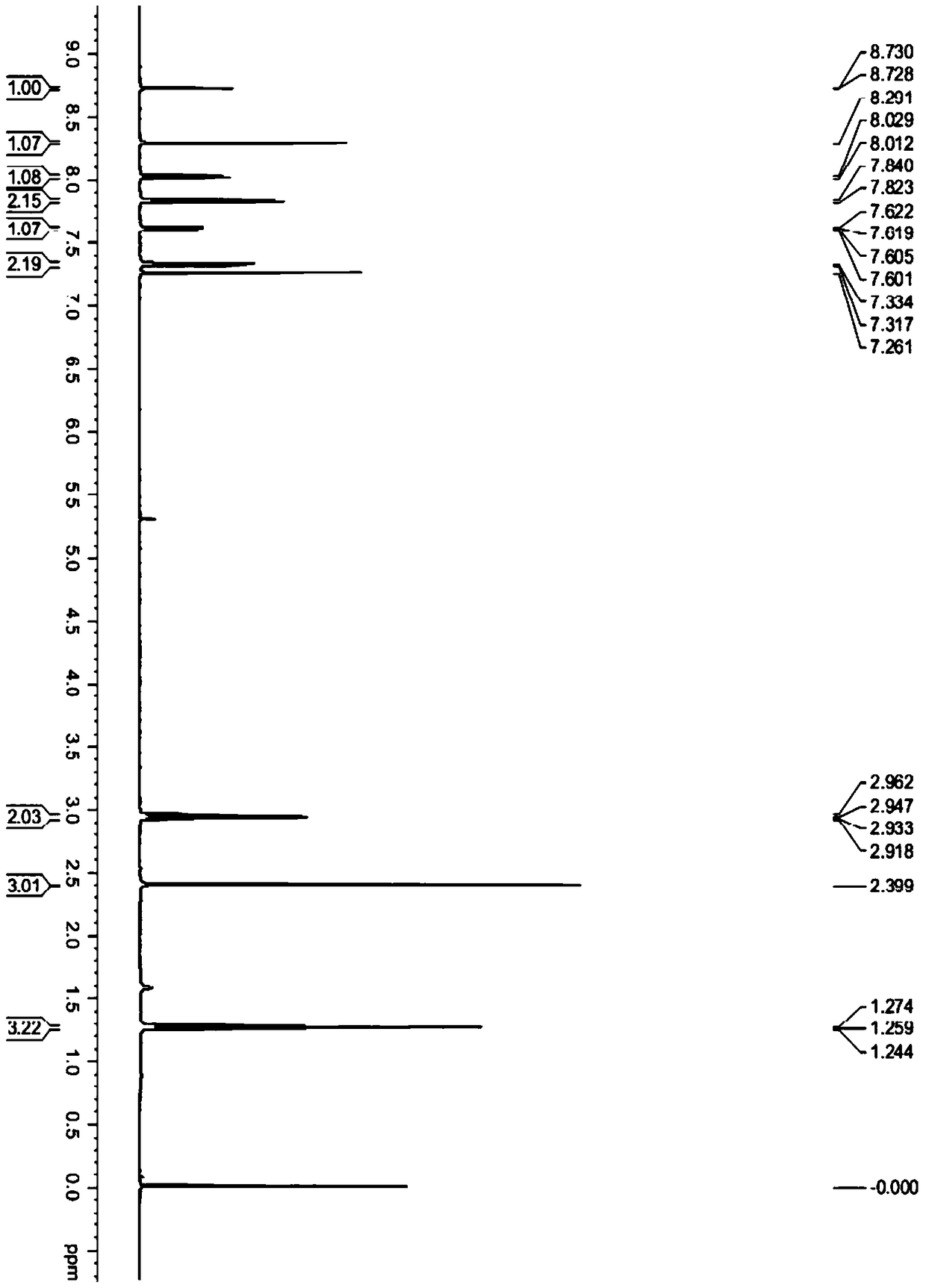
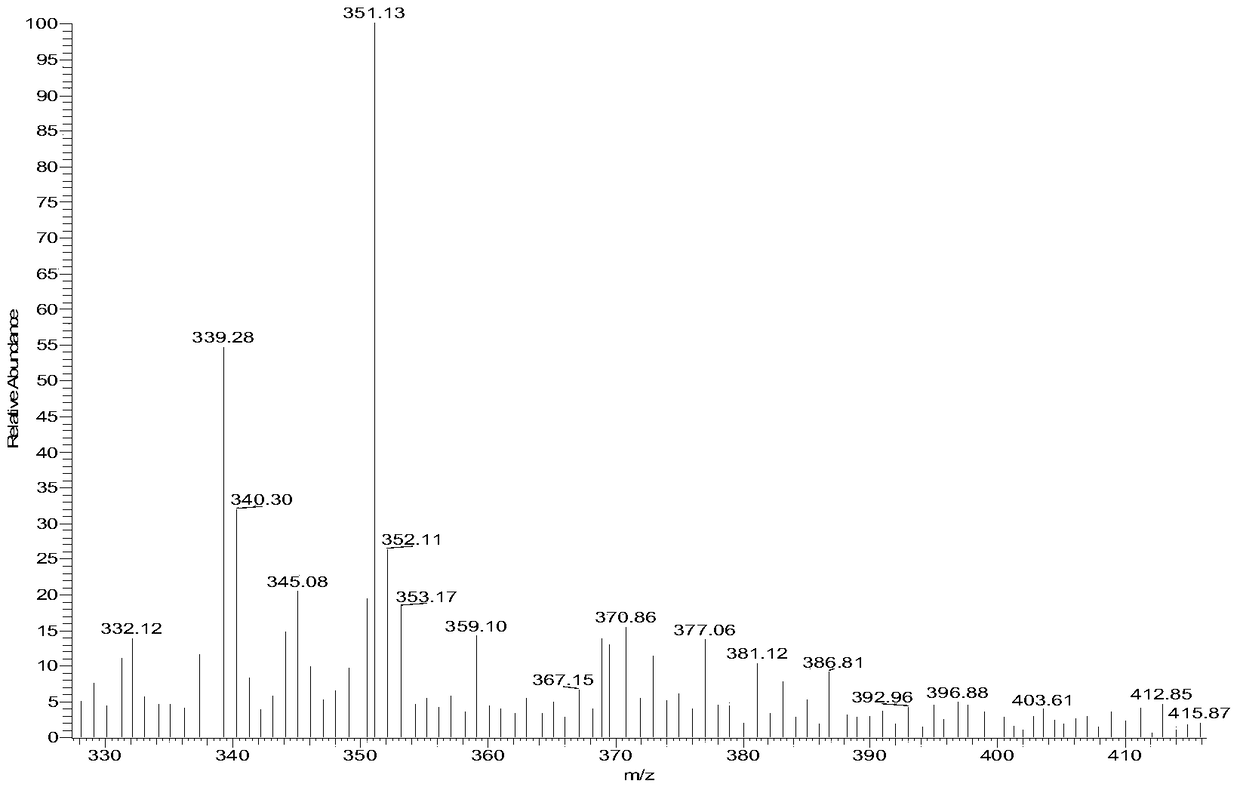
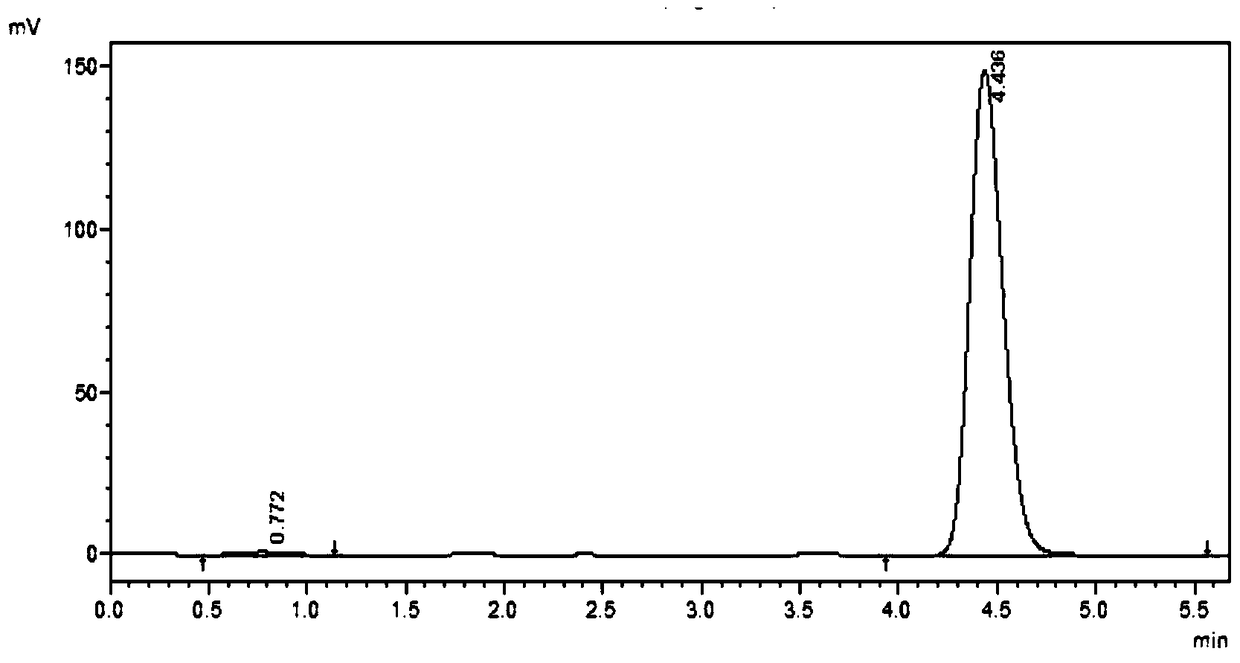
N-substituted benzenesulfonyl-3-propionyl indole derivatives, preparation method and application
A technology of propionyl indole and benzenesulfonyl, which is applied in the field of organic synthesis and achieves the effects of easy operation and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Compound 16:
[0044]
[0045] Its preparation method is:
[0046] Weigh 6-methylindole (1mmol), p-toluenesulfonyl chloride (1.2mmol), sodium hydroxide (1.8mmol) and TEBA (0.1mmol) in a 50mL flask, add dichloromethane (5mL), room temperature React for 1.5h, TLC tracking detection until the reaction is complete, add water (10mL) to stop the reaction, extract with (20mL×3 times) dichloromethane, combine the organic phase, wash the organic phase with saturated sodium chloride solution (10mL), anhydrous sulfuric acid It was dried over sodium and concentrated under reduced pressure to obtain compound 16, which was directly used to prepare compound 1 without isolation.
[0047] The reaction formula for preparing compound 1 from compound 16 is:
[0048]
[0049] Its preparation method is:
[0050] 3mmol of AlCl 3Add to a 50mL flask and add 5mL of dichloromethane, then add 1.5mmol of propionyl chloride at room temperature, after 15 minutes of reaction (that is, after ...
Embodiment 2
[0057] Compound 17:
[0058]
[0059] Its preparation method is:
[0060] Weigh 5-cyanindole (1mmol), p-toluenesulfonyl chloride (1.2mmol), sodium hydroxide (1.8mmol) and TEBA (0.1mmol) in a 50mL flask, add dichloromethane (5mL), room temperature React for 2 hours, TLC tracking detection until the reaction is complete, add water (10mL) to terminate the reaction, extract with (20mL×3 times) dichloromethane, combine the organic phase, wash the organic phase with saturated sodium chloride solution (10mL), anhydrous sodium sulfate Drying and concentration under reduced pressure gave compound 17, which was directly used to prepare compound 2 without isolation.
[0061] The reaction formula for preparing compound 2 from compound 17 is:
[0062]
[0063] Its preparation method is:
[0064] 3mmol of AlCl 3 Add to a 50mL flask and add 5mL of dichloromethane, then add 1.5mmol of propionyl chloride at room temperature, after 15min of reaction (that is, after the reaction soluti...
Embodiment 3
[0075] Compound 18:
[0076]
[0077] Its preparation method is:
[0078] Weigh 6-methylindole (1mmol), p-ethylbenzenesulfonyl chloride (1.2mmol), sodium hydroxide (1.8mmol) and TEBA (0.1mmol) in a 50mL flask, add dichloromethane (5mL), room temperature React for 1.5h, TLC tracking detection until the reaction is complete, add water (10mL) to stop the reaction, extract with (20mL×3 times) dichloromethane, combine the organic phase, wash the organic phase with saturated sodium chloride solution (10mL), anhydrous sulfuric acid It was dried over sodium and concentrated under reduced pressure to obtain compound 18, which was directly used to prepare compound 3 without isolation.
[0079] The reaction formula for preparing compound 3 from compound 18 is:
[0080]
[0081] Its preparation method is:
[0082] 3mmol of AlCl 3 Add it to a 50mL flask and add 5mL of dichloromethane, then add 1.5mmol of propionyl chloride at room temperature, and after 15min of reaction (that is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com