Aripiprazole long-acting controlled-release microgranule injection and preparation method thereof
A technology of aripiprazole and sustained-release microparticles, which is applied in the field of aripiprazole long-acting sustained-release microparticle injections and its preparation, which can solve the problem that the drug cannot be completely released from the microparticles, and the ideal in vitro drug release behavior cannot be achieved. Slowness and other problems, to achieve the effect of overcoming sudden release, low burst release, and complete release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of Aripiprazole Microparticles:
[0055]
[0056] Dissolve polylactic acid-glycolic acid and aripiprazole with a solvent and a drug release characteristic modifier, then add it to the mixed solution of PVA and water, keep the curing temperature at 0°C, and emulsify at 1000 (rpm) for 30 minutes under magnetic stirring Finally, reduce the rotation speed and stir to 500 (rpm) for 1 hour to volatilize the solvent. After the microparticles are solidified, separate the microparticles and wash the microparticles continuously with sterile water for 8 hours. Filter out and dry in vacuum at room temperature for 24h;
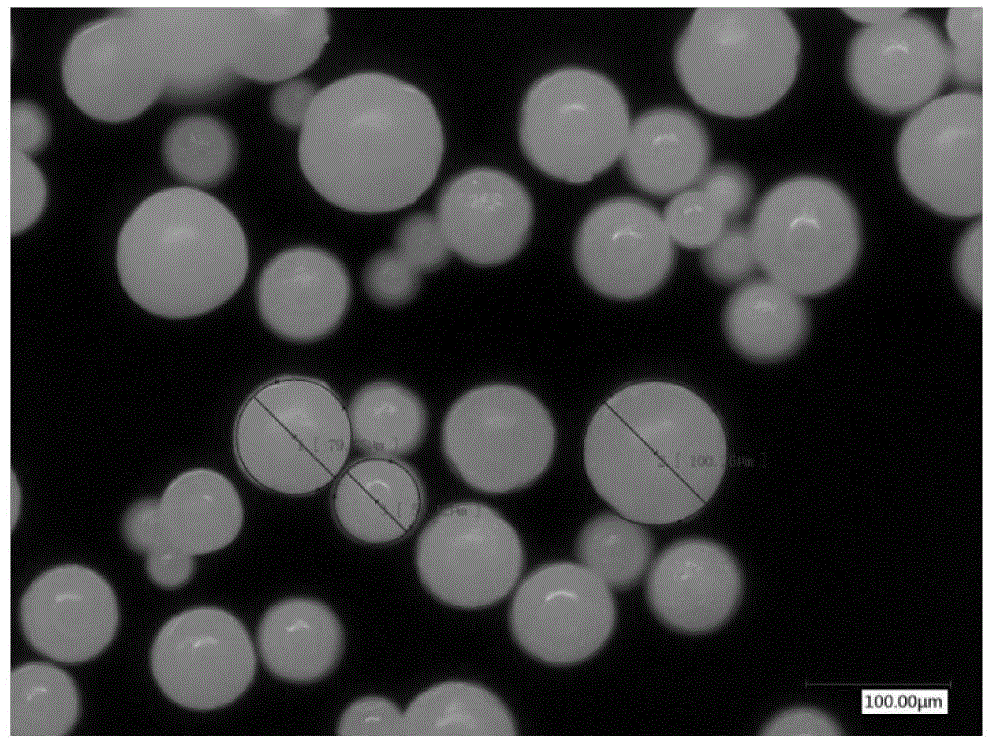
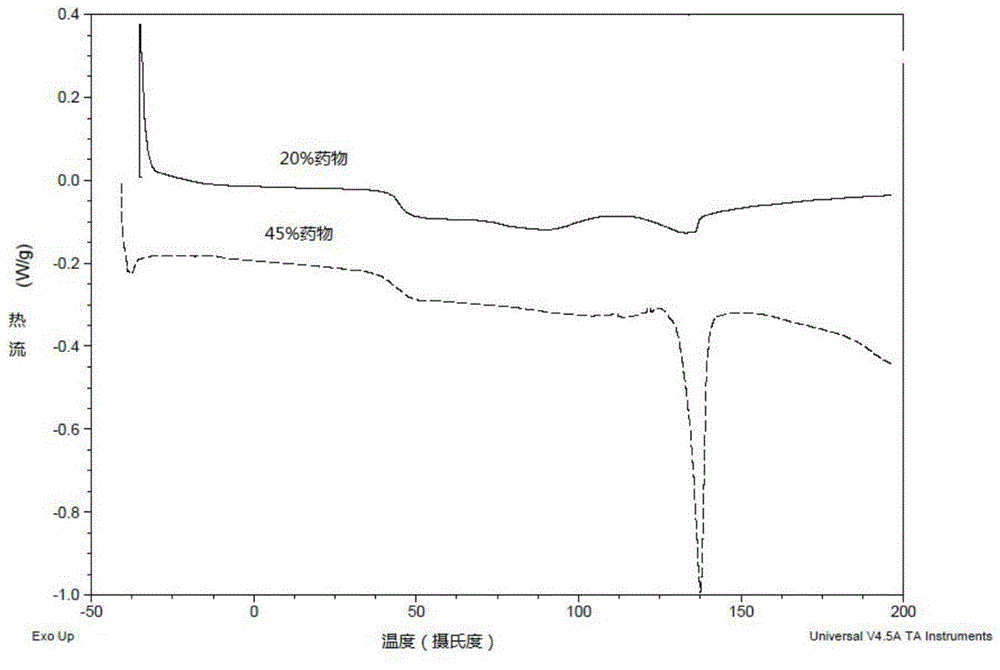
[0057] The diameter of the obtained particles is 73.56±24.34 μm, the drug loading is 35.97%, and the encapsulation efficiency is 89.94%.
[0058] Adopt HPLC and GC method to detect, the weight percent content of each component of particle:
[0059]
[0060]
Embodiment 2
[0062] Preparation of Aripiprazole Microparticles:
[0063]
[0064] Dissolve polylactic acid and aripiprazole with a solvent and a drug release characteristic regulator, then add it to the mixed solution of PVA and water, keep the curing temperature at 5°C, and emulsify at 2000 (rpm) for 15 minutes under magnetic stirring, and then reduce Stir at a rotational speed of 500 (rpm) for 0.5 h to volatilize the solvent. After the microparticles are solidified, separate the microparticles and wash the microparticles continuously with sterile water for 8 hours. Filter out and dry in vacuum at room temperature for 24h;
[0065] The diameter of the obtained particles is 77.02±25.48 μm, the drug loading is 44.80%, and the encapsulation efficiency is 89.17%.
[0066] Adopt HPLC and GC method to detect, the weight percent content of each component of particle:
[0067]
Embodiment 3
[0069] Preparation of Aripiprazole Microparticles:
[0070]
[0071]
[0072] Dissolve polylactic acid-glycolic acid and aripiprazole with a solvent and a drug release characteristic regulator, then add it to the mixed solution of PVA and water, keep the curing temperature at 20°C, and emulsify at 1000 (rpm) for 20 minutes under magnetic stirring Finally, reduce the rotation speed and stir to 500 (rpm) for 1 hour to volatilize the solvent. After the microparticles are solidified, separate the microparticles and wash the microparticles continuously with sterile water for 8 hours. Filter out and dry in vacuum at room temperature for 24h;
[0073] The diameter of the obtained particles is 80.39±20.38 μm, the drug loading is 25.03%, and the encapsulation efficiency is 75.87%.
[0074] Adopt HPLC and GC method to detect, the weight percent content of each component of particle:
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com