Levodropropizine inclusion compound dispersible tablet and preparation method thereof
A levodropropizine and clathrate technology, which is applied in the field of levodropropizine clathrate dispersible tablets and its preparation, can solve problems such as poor taste and reduced bitterness, and achieve reasonable formula, improved taste and stable process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052]Embodiment 1: levodropropizine inclusion compound dispersible tablet,
[0053] Levodropropizine clathrate: the mol ratio of β-cyclodextrin is 1:1;
[0054] The prescription of dispersible tablets is (calculated by mass percentage):
[0055] Levodropropizine-β-cyclodextrin inclusion complex 4g
[0056] L-HPC 0.5g
[0057] Magnesium stearate 0.02g
[0058] Microcrystalline cellulose 0.48g
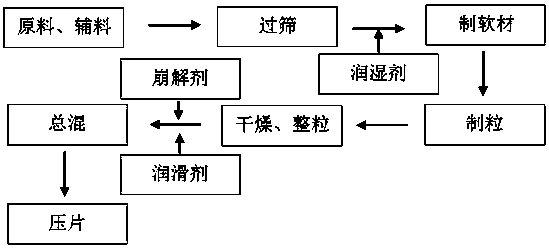
[0059] Preparation:
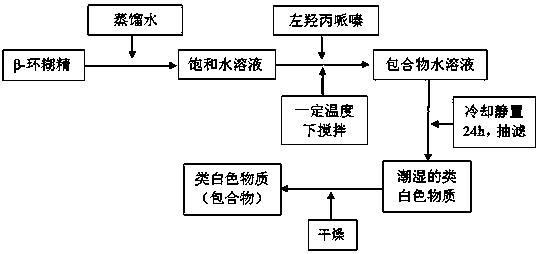
[0060] 1) Prepare the levodropropizine inclusion compound according to the following method: use water as the solvent, react levodropropizine and β-cyclodextrin at a molar ratio of 1:1, the reaction temperature is 65°C, keep stirring , the reaction time was 30min, and the reaction solution was obtained; the obtained reaction solution was refrigerated for 24 hours, and the reaction precipitate was obtained by suction filtration, and the reaction precipitate was washed with absolute ethanol, and the clathrate was separated from the reaction precipitate, and the ...
Embodiment 2
[0063] Levodropropizine clathrate: the mol ratio of β-cyclodextrin is 1:1;
[0064] The prescription for dispersible tablets is (by weight):
[0065] Levodropropizine-β-cyclodextrin inclusion complex 16.12g
[0066] L-HPC 1.8g
[0067] Magnesium stearate 0.08g
[0068] The preparation method is the same as in Example 1.
Embodiment 3
[0070] Levodropropizine inclusion complex: β-cyclodextrin 1:1
[0071] The prescription for dispersible tablets is (by weight):
[0072] Levodropropizine-β-cyclodextrin inclusion compound 322.4g
[0073] L-HPC 36g
[0074] Magnesium stearate 1.6g
[0075] The preparation method is the same as in Example 1.
[0076] The products prepared in the above examples, after investigation, have significantly reduced bitterness.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com